Abstract
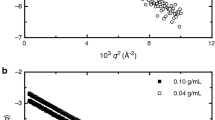
Models for Ph4AsCl (aq.) which incorporate a Gurney cosphere overlap term are fitted to the published osmotic coefficient data. Models with predominantly + − and those with predominantly ++ ion pairing are about equally successful. Further calculations are then made to see how difficult it might be to distinguish between the models by scattering x rays, electrons, or neutrons from the aqueous solution in the rather low concentration range in which the models might be realistic. The x-ray experiment is promising, but high precision in the small-angle region would be necessary.
Similar content being viewed by others
References
P. S. Ramanathan and H. L. Friedman,J. Chem. Phys. 54, 1086 (1971).
H. L. Friedman and P. S. Ramanathan,J. Phys. Chem. 72, 3352 (1968).
P. S. Ramanathan, C. V. Krishnan, and H. L. Friedman,J. Solution Chem. 1, 237 (1972).
W. H. Streng, and W.-Y. Wen,J. Solution Chem. 3, 865 (1974).
H. L. Friedman, A. Smitherman, and R. DeSantis,J. Solution Chem. 2, 59 (1973).
C. V. Krishnan and H. L. Friedman,J. Solution Chem. 3, 727 (1974).
J. C. Rasaiah and H. L. Friedman,J. Chem. Phys. 48, 2742 (1968).
J. C. Rasaiah,J. Chem. Phys. 52, 704 (1970).
H. L. Friedman, L. P. Hwang, and C. V. Krishnan, inStructure of Water and Aqueous Solutions, W. Luck, ed. (Verlag Chemie, 1974), p. 169.
H. L. Friedman and W. D. T. Dale, inModern Theoretical Chemistry, Vol. 5, Bruce Berne, ed. (Plenum Press, New York, 1976), p. 85.
G. Fournet,Encyclopedia of Physics, Vol. 32, S. Flügge, ed. (Springer Verlag, Berlin, 1957), p. 296; J. Waser and V. Schomaker,Rev. Mod. Phys. 25, 671 (1953).
A. H. Narten and H. Levy, inWater: A Comprehensive Treatise, Vol. 1, F. Franks, ed. (Plenum Press, New York, 1972), Chap. 8.
L. Blum and A. H. Narten,Adv. Chem. Phys. 34, 203 (1976).
D. I. Page and K. Mika,J. Phys. C 4, 3034 (1971).
J. T. Enderby,Chem. Phys. Lett. 21, 109 (1953).
E. Kalman and G. Palinkas, On the Role of Electron Diffraction Among Diffraction Studies on Water Structure, Collected abstracts, Second European Crystallographic Meeting, Keszthely (1974), p. 494.
D. P. Wilson and W.-Y. Wen,J. Phys. Chem. 79, 1527 (1975).
International Tables for X-Ray Crystallography, Vol. 3, K. Lonsdale, ed. (The Kynoch Press, Birmingham, England, 1969); P. A. Doyle and P. S. Turner,Acta Cryst. A24, 390 (1968).
G. Kalfoglu and L. H. Bowen,J. Phys. Chem. 73 2728 (1969).
H. L. Friedman,J. Solution Chem. 1, 387 (1972).
R. M. Diamond,J. Phys. Chem. 67, 2513 (1963).
W. Kauzmann,Adv. Protein Chem. 14, 1 (1959).
L. S. Ornstein and F. Zernike,Versl. Akad. Wetenschap. 23, 582 (1914).
B. Conway, J. E. Desnoyers, and R. E. Verrall,Trans. Faraday Soc. 62, 2738 (1966).
H. L. Friedman and C. V. Krishnan,J. Phys. Chem. 78, 1927 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Friedman, H.L., Zebolsky, D.M. & Kalman, E. Calculated X-ray scattering functions for models for aqueous Ph4AsCl which fit the osmotic coefficient data. J Solution Chem 5, 853–865 (1976). https://doi.org/10.1007/BF01167239
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01167239