Abstract
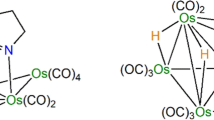
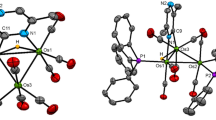
The reaction of [Os3(CO)12] with tetramethylthiourea in the presence of a methanolic solution of Me3NO·2H2O at 60° yields the compounds [Os3(CO)11{η 1-SC(NMe2)2}] (1) in 56% yield and [Os3(CO)9(μ-OH)(μ-MeOCO){η 1-SC(NMe2)2}] (2) in 10% yield in which the tetramethylthiourea ligand is coordinatedvia the sulfur atom at an equatorial position. Compound2 is a 50 e− cluster with two metal-metal bonds and the hydroxy and methoxycarbonyl ligands bridging the open metal-metal edge. In contrast, the analogous reaction of [Os3(CO)12] with thiourea gives the compounts [(μ-H)Os3(CO)10{μ-NHC(S)NH2}] (3) in 8% yield and [(μ-H)Os3(CO)9{3-NHC(S)NH2}] (4) in 30% yield. In3, the thioureato ligand bridges two osmium atomsvia the sulfur atom, whereas in4 in addition to the sulfur bridge, one of the nitrogen atoms of thioureato moiety bonds to the remaining osmium atom. The decacarbonyl compounds 3 can also be obtained in 50% yield from the reaction of [Os3(CO)10(MeCN)2] with thiourea at ambient temperature. Compound3 converts to4 (65%) photochemically. Compound1 reacts with PPh3 and acetonitrile at ambient temperature to give the simple substitution products [Os3(CO)11(PPh3)] and [Os3(CO)11(MeCN)], respectively, while with pyridine, the oxidative addition product [(μ-H)Os3(CO)10(μ-NC5H4] is formed at 80°C. All the new compounds are characterized by IR,1-H-NMR and elemental analysis together with the X-ray crystal structures of1,2 and4. Compound1 crystallizes in the triclinic space group P\(P\bar 1\)with unit cell parametersa = 8.626(3) Å,b = 11.639(3) Å,c = 12.568(3_ Å,α = 84.67(2)°,β = 75.36(2)°,γ = 79.49(3)°,V = 1199(1) Å3, andZ = 2. Least-squares refinement of 4585 reflections gave a final agreement factor ofR = 0.0766 (R w = 0.0823). Compound2 crystallizes in the monoclinic space group P21/n with unit cell parametersa = 9.149(5) Å,b = 17.483(5) Å,c = 15.094(4) Å,β = 91.75(2)°,V = 2413(2) Å3, andZ = 4. Least-squares refinement of 3632 reflections gave a final agreement factor ofR = 0.0603 (R w = 0.0802). Compound4 crystallizes in the monoclinic space group C2/c with unit cell parametersa = 13.915(7) Å,b = 14.718(6) Å,c = 17.109(6) Å,β = 100.44(3)°,V = 3446(5) Å3, andZ = 8. Least-squares refinement of 2910 reflections gave a final agreement factor ofR = 0.0763 (R w = 0.0863).
Similar content being viewed by others
References
U. Bodensieck, H. Stoeckli-Evans, and G. Süss-Fink (1990).Chem. Ber. 123, 1603.
U. Bodensieck, J. Santiago, H. Stoeckli-Evans, and G. Süss-Fink (1992).J. Chem. Soc., Dalton Trans. 255.
U. Bodensieck, H. Stoeckli-Evans, and G. Süss-Fink (199).J. Chem. Soc., Chem. Commun. 267.
U. Bodensieck, H. Stoeckli-Evans, and G. Süss-Fink (1992).J. Organomet. Chem. 433, 149; (b) U. Bodensieck, H. Stoeckli-Evans, and G. Süss-Fink (1992).J. Organomet. Chem. 433, 167.
U. Bodensieck, L. Hoferkamp, H. Stoeckli-Evans, G. Rheinwald, and G. Süss-Fink (1993).J. Chem. Soc., Dalton Trans., 127.
E. Boroni, G. Predieru, A. Tirripicchio, and M. Tirripicchio Camellini (1993).J. Organometal. Chem. 451, 163.
E. W. Ainscough, A. M. Brodie, S. L. Ingham, T. G. Kotch, A. J. Lees, L. Lewis, and J. M. Waters (1994).J. Chem. Soc., Dalton Trans. 1.
J. A. Clucas, D. F. Foster, M. M. Harding, and A. K. Smith (1984).J. Chem. Soc., Chem. Commun. 949; (b) S. Cartwright, J. A. Clucas, R. H> Dawson, D. F. Foster, M. M. Harding, and A. K. Smith (1986).J. Organometal. Chem. 302, 403.
M. R. Churchill and B. G. DeBoer (1977).Inorg. Chem. 16, 828.
R. D. Adams and M. P. Pompeo (1990).Organometallics 9, 1718.
R. D. Adams, N. M. Adams, N. M. Golembeski, and J. P. Selegue (1981).J. Am. Chem. Soc. 103, 546; (b) R. D. Adams, Z. Dawoodi, D. F. Foust, and B. E. Segmüller (1983).Organometallics 2, 315; (c) R. D. Adams, D. A. Katahira, and L. W. Yang (1982).Organometallics 1, 235.
H. D. Holden, B. F. G. Johnson, J. Lewis, P. R. Raithby, and G. Uden (1983).Acta Crystallogr. Sec. C39, 1200; (b) A. M. Brodie, H. D. Holden, J. Lewis, and M. J. Taylor (1986).J. Chem. Soc., Dalton Trans. 633.
V. F. Allen, R. Mason, and P. B. Hitchcock (1977).J. Organomet. Chem. 140, 297.
A. Mangia and G. Pelizzi (1973).Cryst. Struct. Commun. 2, 77.
A. C. Bonamartini, A. Mangia, and G. Pelizzi (1973)Cryst. Struct. Commun. 2, 73.
M. B. Ferrari, A. B. Corradi, G. Fava, C. G. Palmieri, M. Nardelli, and C. Pelizzsi (1973).Acta Crystallogr. B29, 1808.
C. M. Jensen, C. B. Knobler, and H. D. Kaesz (1984).J. Am. Chem. Soc. 106, 5926; (b) C. M. Jensen, T. J. Lynch, C. B. Knobler, and H. D. Kaesz (1982).J. Am. Chem. Soc. 104, 4679.
A. J. Arce, P. Arrojo, A. J. Deeming, and Y. De Sanctis (1991).J. Chem. Soc., Chem. Commun. 1491.
B. F. G. Johnson, J. Lewis, P. R. Raithby, and T. I. Odiaka (1981).J. Organomet. Chem. 216, C56.
G. Ferraris and G. Gervasio (1974)J. Chem. Soc., Dalton Trans. 1813.
C. G. Pierpont (1977).Inorg. Chem. 16, 636.
A. J. Deeming, S. Hasso, P. J. Manning, K. Henrick, and M. McPartlin (1982).J. Chem. Soc., Dalton Trans. 899.
A. J. Deeming, P. J. Manning, L. P. Rothwell, M. B. Hursthouse, and N. P. C. Walker (1984).J. Chem. Soc., Dalton Trans. 2039.
S. R. Hodge, B. F. G. Johnson, J. Lewis, and P. R. Raithby (1987).J. Chem. Soc., Dalton Trans. 931.
A. J. Deeming (1986)_Adv. Organomet. Chem. 26, 1.
P. C. Ford and A. Rokicki (1988).Adv. Organomet. Chem. 28, 139.
E. Rosenberg, S. E. Kabir, K. I. Hardcastle, M. Day, and E. Wolf (1990)Organometallics 9, 2214; (b) S. E. Kabir, M. Day, M. Irving, T. McPhillips, H. Minassian, E. Rosenberg, and K. I. Hardcastle (1991).Organometallics 10, 3997.
M. I. Bruce, D. C. Kehoe, J. G. Matisons, B. K. Nicholson, P. H. Rieger, and M. L. Williams (1982).J. Chem. Soc., Chem. Commun. 442.
B. F. G. Johnson, J. Lewis, and D. A. Pippard (1981).J. Chem. Soc., Dalton Trans. 407.
C. C. Yin, and D. J. Deeming (1975).J. Chem. Soc., Dalton Trans. 2091.
A. G. Yin, Orpen (1980).J. Chem. Soc., Dalton Trans. 2509.
G. W. Frank and P. Degen (1973).Acta Crystallogr. B29, 1815; (b) G. Vallee, V. Busetti, M. Mammi, and G. Carrazolo (1969).Acta Crystallogr. B25, 1432; (c)_ J. E. Fleming and H. Lynton (1967).Can. J. Chem. 45, 353.
P. Piraino, G. Bruno, G. Tresoldi, G. Faraone, and G. Bombieri (1983).J. Chem. Soc., Dalton Trans. 2391.
B. A. Cartwright, P. O. Langguth, Jr., and A. C. Skapski (1979).Acta Crystallogr. B35, 63.
U. Bodensieck, Y., Carraux, H. Stoeckli-Evans, and G. Süss-Fink (1992).Inrog. Chim. Acta. 195, 135.
G. M. Sheldrick (1990).Acta Crystallogr. A46, 467.
C. K. Fair,MOLEN, An Interactive Structure Solution Procedure (Enraf-Nonius, Delft, The Netherlands (1990).
D. T. Cromer and J. T. Waber,International Tables fo X-Ray Crystallography Vol. IV, Table 2.2B (Kynoch Press Birmingham, England, 1974).
D. T. CromerInternational Tables for X-ray Crystallography, Vol. IV, Table 2.3.1 (Kynoch Pres Birmingham, England, 1974).
J. A. Darr, S. R. Drake, M. B. Hursthouse, and K. M. A. Malik (1993).Inorg. Chem. 32, 5704.
G. M. SheldrickSHELXL-93 Program for Crystal Structure Refinement (University of Göttingen, Germany, 1993).
N. P. C. Walker and D. Stuart (1983).Acta Crystallogr. A39, 158 (adapted for FAST geometry by A. Karaulov, University of Wales, 1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Azam, K.A., Dilshad, R., Kabir, S.E. et al. Triosmium clusters derived from the reactions of thioureas with dodecacarbonyltriosmium: Crystal structures of [Os3(CO)11{η 1-SC(NMe2)2}], [Os3(CO)9(μ-OH)(μ-OMeOCO){η 1-SC(NMe2)2}] and [(μ-H)Os3(CO)9{μ 3-NHC(S)NH2}]. J Clust Sci 7, 49–70 (1996). https://doi.org/10.1007/BF01166176
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01166176