Abstract
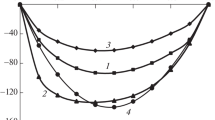
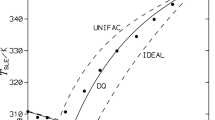
Densities and vapor-liquid equilibrium were determined for 1-chlorobutane and pyridine with 1,1,1-trichloroethane at 25°C. From the experimental results, excess molal volumes and excess molar Gibbs energies were calculated. Information could be obtained about the possible interactions between the components of both binary systems. The Prigogine-Flory-Patterson theory was applied to calculate excess molar volumes. Liquid activity coefficients were calculated and correlated with different expressions existing in the literature.
Similar content being viewed by others
References
S. M. Bardavid, G. C. Pedrosa, and M. Katz,Lat. Am. Appl. Res. 22, 225 (1992).
I. L. Acevedo, E. L. Arancibia, and M. KatzJ. Solution Chem. 22, 191 (1993).
H. T. Van and D. Patterson,J. Solution Chem. 11, 798 (1984).
M. Costas and D. Patterson,J. Solution Chem. 11, 807 (1984).
P. J. Flory,Dis. Faraday Soc. 49, 7 (1970).
P. St. Roman, H. T. Van, and D. Patterson,J. Chem. Soc. Faraday Trans. I,75, 1700 (1979).
V. Lakshman, M. V. Rao and D. H. L. Prasad,Fluid Phase Eq. 69, 271 (1991).
R. K. Kumar, M. V. Rao and D. H. L. Prasad,Fluid Phase Eq. 70, 19 (1991).
A. Lainez, M. M. Rodrigo, W. Wielhelm, and J. P. E. Grolier,J. Solution Chem. 21, 49 (1992).
J. Gmehling,J. Chem. Eng. Data 38 143 (1993).
T. Boublik and G. C. Benson,Can. J. Chem. 47, 539 (1969).
J. I. Riddick, N. B. Bunger and T. Sakano,Organic Solvents, 4o Ed., J. Wiley & Sons, New York, (1986).
M. A. M. Saucet, J. José, and C. M. Saucet,Thermochim. Acta. 102, 27 (1986).
V. Srinivasulu and P. R. Naidu,Phys. Chem. Liq. 19, 151 (1989).
I. Prigogine,The Molecular Theory of Solutions, North Holland, Amsterdam, 1957.
D. Patterson and G. Delmas,Dis. Faraday Soc. 49, 98 (1970).
P. J. Flory, R. A. Orwoll and A. Vrij,J. Am. Chem. Soc. 86, 3507 (1964).
J. A. Salas, B. O'Donell, L. Scida and M. Katz,Anal. Assoc. Quim. Arg. 76, 427 (1988).
J. G. Hayden and J. P. O'Connell,Ind. Eng. Chem. Process Des. Dev. 14, 209 (1975).
A. Fredenslund, J. Gmehling and P. Rasmussen,Vapor Liquid Equilibria using UNIFAC, Elsevier, Amsterdam (1977).
J. M. Prausnitz,Molecular Thermodynamics of Fluid Phase Equilibria, Prentice Hall, Inc., N.J. (1969).
A. G. Wilson,J. Am. Chem. Soc. 86, 127 (1964).
H. Renon and J. M. Prausnitz,A. I. Ch. E. J. 14, 135 (1968).
D. S. Abrams and J. M. Prausnitz,A. I. Ch. E. J. 21, 116 (1978).
J. Gmehling, P. Rasmussen and A. Fredenslund,Ind. Eng. Chem. Process Des. Dev. 21, 116 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Salas, J.A., Katz, M. Excess molar volumes and isothermal vapor-liquid equilibrium in the binary system 1,1,1-trichloroethane with 1-chlorobutane and with pyridine at 25°C. J Solution Chem 24, 357–367 (1995). https://doi.org/10.1007/BF01150874
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01150874