Abstract
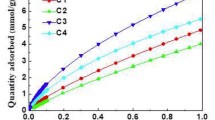
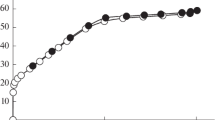
The characterization of the pore structure of microporous materials is of interest because of the usefulness of these materials in many applications. Of these, the characterization of carbon adsorbents is particularly problematic because of the presence of small pores with size on the order of small molecules (micropores) along with a wide distribution of pore sizes, and their non-crystalline structure. In this paper, we present results obtained using the Dubinin-Astakhov equation to analyze data from high pressure CO2 adsorption at 273 K to characterize two sets of microporous carbons. Our results support the conclusions of previous workers that the Dubinin-Astakhov (DA) equation is able to linearize adsorption data that gives rise to curved Dubinin-Radushkevich plots. However, when applied over different ranges of relative pressure on the adsorption isotherm, the Dubinin-Astakhov plots result in different values of micropore volume and characteristic adsorption potential. Furthermore, DA analysis of CO2 (273 K) adsorption data over a wide range of pressures (10−3–22000 Tort), gives results different from DA analysis of CO2 (273 K) isotherms measured at low pressures only (10−3–830 Tort). It would appear desirable to apply the DA equation to data that reflects the entire range of micropore filling on the adsorption isotherm, as opposed to data over a limited relative pressure range. For CO2 adsorption at 273 K, this would necessitate adsorption studies at high pressures, to about 28 atm. Micropore volumes obtained in this manner, agreed with the total pore volumes determined by nitrogen (77 K) adsorption for all the activated carbons studied.
Similar content being viewed by others
References
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, and T. Siemienewska, Pure & Appl. Chem.57, 603 (1985).
H. Juntgen, Carbon15, 2731 (1977).
R.V. Carrubba, J.E. Urbanic, N.J. Wagner, and R.H. Zanitsch, AIChE Symposium Series (Adsorption and Ion Exchange)80(233), 76.
R.C. Bansal, J.P. Donnet, and F. Stoeckli, inActive Carbon (Marcel Dekker, New York, 1988).
S.J. Gregg and K.S.W. Sing, inAdsorption, Surface Area, and Porosity (Academic Press, London, 1982).
B.C. Lippens, B.G. Linsen, and J.H. de Boer, J. Catalysis3, 32 (1964).
K.S.W. Sing, inSurface Area Determination, Procedings of an International Symposium, 1969, edited by D.H. Everett and R.H. Ottewill (Butterworths, London, 1970), p. 25.
M.M. Dubinin, inProgress in Surface and Membrane Science, edited by D.A. Cadenhead, J.F. Danielli, and M.D. Rosenberg (Academic Press, New York, 1975), Vol. 9.
R. Ghosal, Y.W. Kim, D.W. Hua, J.V. D'Souza, W. Earl, and D.M. Smith, (manuscript in preparation).
M. Polanyi, Trans. Faraday Soc.28, 316 (1932).
M.M. Dubinin, E.D. Zaverina, and L.V. Radushkevich, Zh. Fiz Khimii21, 1351 (1947).
B. McEnaney, Carbon25, 69 (1987).
B. McEnaney, Carbon26, 267 (1988).
H. Marsh, Carbon25, 49 (1987).
I.M.K. Ismail, Carbon29, 119 (1991).
H. Marsh and B. Rand, J. Coll. Interface Sci.33, 101 (1970).
B. Rand, J. Coll. Interface Sci.56, 337 (1976).
E.M. Freeman, T. Siemienewska, H. Marsh, and B. Rand, Carbon8, 7 (1970).
F. Carrasco-Marin, M.V. Lopez-Ramon, and C. Moreno-Castilla, Langmuir9, 2758 (1993).
F. Rodriguez-Reinoso and A. Linares-Solano, inChemistry & Physics of Carbon, edited by P.A. Thrower (Marcel Dekker, 1989), Vol. 21.
J. Garrido, A. Linares-Solano, J.M. Martin-Martinez, M. Molina-Sabio, F. Rodriguez-Reinoso, and R. Torregrosa, Langmuir3, 76 (1987).
H. Marsh and W.T.K. Wynne-Jones, Carbon1, 269 (1964).
T.G. Lamond and H. Marsh, Carbon1, 281 (1964).
P. Gonzalez-Vilchez, A. Linares-Solano, J.D. Lopez-Gonzalez, and F. Rodriguez-Reinoso, Carbon17, 441 (1979).
J.D. Lopez-Gonzalez, F. Martinez-Vilchez, and F. Rodriguez-Reinoso, Carbon18, 413 (1980).
E. Maglara, A. Pullen, D. Sullivan, and W.C. Conner, Langmuir, (in press).
R. Ghosal, MS Thesis, University of New Mexico (1994).
C.G.V. Burgess and D.H. Everett, J. Coll. Interface Sci.33, 611 (1970).
M. Kobayashi, E. Ishikawa, and Y. Toda, Carbon29, 677 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ghosal, R., Smith, D.M. Micropore characterization using the Dubinin-Astakhov equation to analyze high pressure CO2 (273 K) adsorption data. J Porous Mater 3, 247–255 (1996). https://doi.org/10.1007/BF01137914
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01137914