Summary
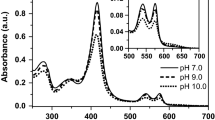
Vitreoscilla contained a homodimeric bacterial hemoglobin (VtHb). The purification of this protein yielded VtmetHb which exhibited electronic and electron paramagnetic resonance (EPR) spectra, showing that it existed predominantly in a high-spin ferric form, both axial and rhombic components being present. The preparations also contained variable amounts of low-spin components. There was no evidence that these high-spin and low-spin forms were in equilibrium. The former were reducible by NADH catalyzed by the NADH-metVtHb reductase, and the latter were not. High ionic strength and high pH led to the formation of low-spin metVtHb; both treatments were reversible. Cyanide and imidazole liganded to VtHb resulted in the conversion of high-spin to low-spin ferric heme centers, each with characteristic electronic and EPR spectra. Some preparations of VtHb exhibited EPR signals consistent with a sulfur ligand bound to the ferric site. When VtHb was treated with NADH plus the reductase in the presence of oxygen, the intensity of the high-spin EPR signals decreased significantly. No reduction occurred in the absence of oxygen, suggesting a possible role for the superoxide anion. Dithionite treatment of VtHb resulted in a slow reduction, but the main product of the reaction of dithionite-reduced VtHb with oxygen was VtmetHb, not VtHbO2. EPR spectra of whole cells ofVitreoscilla exhibited a variety of intense signals at low and high magnetic field, theg-values being consistent with the presence of high-spin ferric heme proteins, in addition to an iron-containing superoxide dismutase (FeSOD) and iron-sulfur proteins. EPR spectra of the cytosol fraction ofVitreoscilla showed the expected resonances for VtmetHb and FeSOD.
Similar content being viewed by others
Abbreviations
- A :
-
absorbance
- DEAE:
-
diethylaminoethyl
- EDTA:
-
ethylenediamine tetraacetate
- EPR:
-
electron paramagnetic resonance
- HiPIP:
-
high-potential iron protein
- SDS:
-
sodium dodecyl sulfate
- SOD:
-
superoxide dismutase
- VtHb:
-
Vitreoscilla hemoglobin
- VtmetHb:
-
oxidizedVitreoscilla hemoglobin
- VtHbO2 :
-
oxygenatedVitreoscilla hemoglobin
References
Aasa R (1970) Powder line shapes in the electron paramagnetic resonance spectra of high-spin ferric complexes. J Chem Phys 52:3919–3930
Aasa R, Vaenngard T (1975) EPR signal intensity and powder shapes: reexamination.J Magn Reson 19:308–315
Asada K, Yoshikawa K, Takahashi M, Maeda Y, Enmanji K (1975) Superoxide dismutase from a blue-green algae,Plectonema boryanum. J Biol Chem 250:2801–2807
Bache R, Kroneck PMH, Merkle H, Beinert H (1983) A survey of EPR-detectable components in sulfur-reducing bacteria. Biochim Biophys Acta 722:417–426
Bayer E, Hill HAO, Roeder A, Williams RJP (1969) The interaction between haem-iron and thiols. Chem Commun: 109
Beinert H, Orme-Johnson WH, Palmer G (1978) Special techniques for the preparation of samples for low-temperature EPR spectroscopy. Methods Enzymol 54:111–132
Blumberg WE, Peisach J (1971) A unified theory for low-spin forms of all ferric heme proteins as studied by EPR. In: Chance B, Yonetani T, Mildvan AS (eds) Probes of structure and function of macromolecules and membranes. Vol. II. Probes of enzymes and hemoproteins. Academic Press, New York, London, pp 215–229
Bohan TL (1977) Analysis of low-spin ESR spectra of ferric heme proteins: a reexamination. J Magn Reson 26:109–118
Choc MG, Webster DA, Caughey WS (1982) Oxygenated intermediate and carbonyl species of cytochromeo (Vitreoscilla). J Biol Chem 257:865–869
Dawson JH, Andersson LA, Sono M (1982) Spectroscopic investigations of ferric cytochromeP-450cam ligand complexes. Identification of the ligandtrans to cysteinate in the native enzyme. J Biol Chem 257:3606–3617
De Vries S, Albracht S (1979) Intensity of highly anisotropic lowspin heme EPR signals. Biochim Biophys Acta 546:334–340
Georgiou CD, Webster DA (1987a) Identification ofb, c, andd cytochromes in the membrane ofVitreoscilla. Arch Microbiol 148:328–333
Georgiou CD, Webster DA (1987b) Purification and partial characterization of the membrane-bound cytochromeo (561, 564) fromVitreoscilla. Biochemistry 26:6521–6526
Gonzales-Prevatt V, Webster DA (1980) Purification and properties of NADH-cytochromeo reductase fromVitreoscilla. J Biol Chem 255:1478–1482
Guengerich FP, MacDonald TL (1984) Chemical mechanisms of catalysis by cytochromeP-450: a unified view. Acc Chem Res 17:9–16
Harris-Loew GM (1970) An analysis of the electron spin resonance of low-spin ferric heme compounds. Biophys J 10:196–212
Hatchikan EC, Henry YA (1977) An iron-containing superoxide dismutase from the strict anaerobeDesufovibrio desulfuricans (Norway 4). Biochimie 59:153–161
Jakob W (1984) Untersuchungen zur Struktur und Funktion sauerstoffaktivierender Eisen- und Kupferproteine: 1. Ascorbat Oxidase ausCurcurbita pepo medullosa 2. Cytochromo/NADH-Cytochromo Reduktase ausVitreoscilla. Dissertation Universität Konstanz
Kirby TW, Lancaster JR, Fridovich I (1981) Isolation and characterization of the iron-containing superoxide dismutase fromMethanobacterium bryantii. Arch Biochem Biophys 210:140–148
Lipscomb JD (1980) Electron paramagnetic resonance detectable states of cytochromeP-450cam. Biochemistry 19:3590–3599
Liu CY, Webster DA (1974) Spectral characteristics and interconversions of the reduced, oxidized and oxygenated forms of purified cytochromeo. J Biol Chem 249:4261–4266
Orii Y, Webster DA (1977) Oxygenated cytochromeo (Vitreoscilla) formed by treating oxidized cytochrome with superoxide anion. Plant Cell Physiol 18:521–526
Orii Y, Webster DA (1986) Photodissociation of oxygenated cytochromeo (Vitreoscilla) and kinetic studies of reassociation. J Biol Chem 261:3544–3547
Peisach J, Blumberg WE (1971) Departures from axial symmetry as measured by EPR for high spin ferric heme proteins. In: Chance B, Yonetani T, Mildvan AA (eds) Probes of structure and function of macromolecules and membranes. Vol. II. Probes of enzymes and hemoproteins. Academic Press, New York, London, pp 231–239
Peisach J, Blumberg WE (1972) Low-temperature EPR studies of the effects of protein conformation on the symmetry of heme in high-spin ferriheme proteins. In: Åkeson A, Ehrenberg A (eds) Structure and function of oxidation reduction enzymes. Academic Press, New York, San Francisco, London, pp 191–203
Peisach J, Blumberg WE, Adler A (1973) Electron paramagnetic resonance studies of iron porphyrin and chlorin systems. Ann NY Acad Sci 206:310–327
Perutz MF (1978) Hemoglobin structure and respiratory transport. Sci Am 239:92–125
Perutz MF (1980) Stereochemical mechanism of oxygen transport by haemoglobin Proc Roy Soc Lond Ser B 208:135–162
Poulos T, Finzel BC, Gunsalus IC, Wagner GC, Kraut J (1985) The 2.6-Å crystal structure ofPseudomonas putida cytochromeP-450. J Biol Chem 260:16122–16130
Riester J, Zumft WG, Kroneck PMH (1989) Nitrous oxide reductase fromPseudomonas stutzeri. Redox properties and spectroscopic characterization of different forms of the multicopper enzyme. Eur J Biochem 178:751–762
Ruf HR, Ahr H, Nastainczyk W, Ullrich V, Mansuy D, Battioni J-P, Montiel-Montoya R, Trautwein A (1984) Formation of a ferric carbanion complex from halothane and cytochromeP-450: Electron spin resonance, electronic spectra, and model complexes. Biochemistry 23:5300–5306
Tyree B, Webster DA (1978a) The binding of cyanide and carbon monoxide to cytochromeo purified fromVitreoscilla. Evidence for subunit interaction in the reduced protein. J Biol Chem 253:6988–6991
Tyree B, Webster DA (1978b) Electron-accepting properties of cytochromeo purified fromVitreoscilla. J Biol Chem 253:7635–7637
Tyree B, Webster DA (1979) Intermediates in the reaction of reduced cytochromeo (Vitreoscilla) with oxygen. J Biol Chem 254:176–179
Wakabayashi S, Matsubara H, Webster DA (1986) Primary sequence of a dimeric bacterial haemoglobin fromVitreoscilla. Nature 322:481–483
Walker FA, Reis D, Balke VL (1984) Models of cytochromesb. 5. EPR studies of low-spin iron(III) tetraphenylporphyrins. J Am Chem Soc 106:6888–6898
Webster DA (1987) Structure and function of bacterial hemoglobin and related proteins. In: Eichhorn GL, Marzilli L (eds). Vol. 7. Elsevier Science Publishing Co., New York, pp 245–265.
Webster DA, Liu CY (1974) Reduced nicotinamide adenine dinucleotide cytochromeo reductase associated with cytochromeo purified fromVitreoscilla. J Biol Chem 249:4257–4260
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kroneck, P.M.H., Jakob, W., Webster, D.A. et al. Studies on the bacterial hemoglobin fromVitreoscilla . Biol Metals 4, 119–125 (1991). https://doi.org/10.1007/BF01135389
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01135389