Conclusions
-
1.
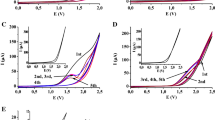
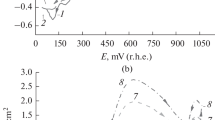
In the oxidation of various organic substances on a smooth platinum electrode, inhibition of the oxidation process is observed in the potential regions 0.55–0.8; 0.9–1.1; 1.3–1.6 V with respect to the normal hydrogen electrode in the same solutions in which a change in the state of the electrode surface as a result of oxygen adsorption is detected by various methods.
-
2.
The characteristic shape of the potentiostatic polarization curves of the oxidation of organic substances and the kinetic inhibition of the oxidation processes result from changes in the state of the electrode surface during the adsorption of oxygen.
-
3.
The rate of the electrooxidation drops exponentially as the amount of adsorbed oxygen increases.
-
4.
In the oxidation of carboxylic acids, alcohols, and aldehydes, the value ofβ is close to 0.5, both for a surface free of oxygen and in the presence of adsorbed oxygen.
Similar content being viewed by others
Literature cited
E. Müller, Z. Elektrochem.,29, 264 (1923);33, 561 (1927).
E. Müller and S. Tanaka, Z. Elektrochem.,34, 256 (1928).
E. Müller and S. Takeganu, Z. Elektrochem.,34, 704 (1928).
S. Tanaka, Z. Elektrochem.,35, 38 (1929).
O. Dany-Hekault, Z. Elektrochem.,6, 533 (1900).
C. Marie and G. Lejenue, J. Chem. Phys.,26, 237 (1929).
A. Hickling and E. J. Rodwell, J. Chem. Soc.,1943, 90.
E. M. Skobets and I. N. Atamanenko, Zavodsk. Laborat.,15, 1291 (1949).
A. I. Shlygin and G. A. Bogdanovskii, Zh. Fiz. Khimii,31, 2428 (1957);34, 57 (1960); Transactions of the Fourth Conference on Electrochemistry [in Russian], Izd. AN SSSR, Moscow (1960), p. 282.
É. A. Aikazyan and Yu. V. Pleskov, Zh. Fiz. Khimii,31, 205 (1957).
K. Schwabe, Z. Elektrochem.,61, 743 (1957).
N. E. Khomutov and S. V. Gorbachev, Zh. Fiz. Khimii,24, 1101 (1950).
V. N. Modestova, Dissertation [in Russian], Moscow (1952).
Yu. B. Vasil'ev and V. S. Bagotskii, Dokl. AN SSSR,148, 132 (1963); Summaries of Reports at the Fourth Conference on the Electrochemistry of Organic Compounds [in Russian], Izd. AN SSSR, Moscow (1962), p. 29; Yu. B. Vasil'ev, Dissertation [in Russian], Moscow (1962).
Yao Lu-An, Yu. B. Vasil'ev, and V. S. Bagotskii, Elektrokhimiya,1, 170 (1965).
P. I. Dolin, D. V. Kokoulina, et al., Dokl. AN SSSR,144, 1081 (1959);147, 649 (1962).
A. N. Frumkin and B. I. Podlovchenko, Dokl. AN SSSR150, 349 (1963).
M. W. Breiter and S. Gilman, J. Elektrochem. Soc.,109, 622, 1099 (1962); Elektrochimica, Acta,7, 533 (1962).
R. P. Buck and L. R. Griffith, J. Elektrochem. Soc.,109, 1005 (1962).
K. I. Rozental' and V. I. Veselovskii, Zh. Fiz. Khimii,27, 1163 (1953);31, 1732 (1957).
V. I. Ginzburg, Zh. Fiz. Khimii,33, 1504 (1959).
G. A. Tedoradze, Zh. Fiz. Khimii,33, 129 (1959).
A. N. Frumkin and É. A. Aikazyan, Dokl. AN SSSR,100, 315 (1955).
É. A. Aikazyan, Zh. Fiz. Khimii,33, 1016 (1959).
T. C. Franklin and S. L. Cooke, J. Electrochem. Soc.,107, 556 (1960).
S. Schuldiner and R. M. Roe, J. Electrochem. Soc.,110, 332 (1963).
S. E. S. El. Wakkad and S. H. Emara, J. Chem. Soc.,1952, 461.
A. N. Frumkin, Zh. Fiz. Khimii,14, 1200 (1940);18, 9 (1949); A. N. Frumkin and A. I. Shlygin, Acta Physicochimica URSS,5, 819 (1936).
A. D. Obrucheva, Zh. Fiz. Khimii,26, 1448 (1952).
K. A. Lezhneva, T. I. Borisova, and M. G. Slin'ko, Kinetika i Kataliz,2, 854 (1961).
T. Erdey-Gruz and I. Yajasdy, Acta chim. Acad. scient, hung.,29, 47 (1961).
F. G. Will and C. A. Knorr, Z. Elektrochem.,64, 258 (1960).
V. I. Ginzburg and V. I. Veselovskii, Zh. Fiz. Khimii,24, 366 (1950); T. I. Borisova and V. I. Veselovskii, Ibid.,27, 1195 (1953).
Ts. I. Zalkind and B. V. Érshler, Zh. Fiz. Khimii,25, 565 (1951).
L. Young, “Anodic oxide films”, Leningrad-New York (1961).
K. I. Vetter and D. Berndt, Z. Elektrochem.,62, 378 (1958).
W. Böld and M. Breiter, Electrochimica Acta,5, 145 (1960); M. W. Breiter, J. Electrochem. Soc.,109, 425 (1962).
M. I. Temkin, Zh. Fiz. Khimii,14, 1153 (1940).
Ya. M. Kolotyrkin, Problemy Fizicheskoi Khimii,1, 81 (1958).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 9, pp. 1531–1539, September, 1965
Rights and permissions
About this article
Cite this article
Khazova, O.A., Vasil'ev, Y.B. & Bagotskii, V.S. Electrooxidation of organic substances on a platinum electrode. Communication 1. General shape of the potentiostatic curves and nature of the inhibition of the electrochemical oxidation processes. Russ Chem Bull 14, 1500–1506 (1965). https://doi.org/10.1007/BF01083788
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01083788