Abstract
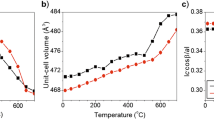
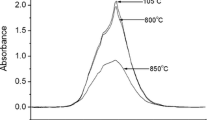
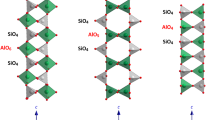
Studies of two muscovites of different iron contents, using solid-state NMR with magic-angle-spinning (MAS) combined with X-ray powder diffraction, thermal analysis and57Fe Mössbauer spectroscopy, suggest that dehydroxylation occurs by a homogeneous rather than an inhomogeneous mechanism, forming a dehydroxylate in which the aluminium is predominantly 5-coordinate. On further decomposition at about 1100° C, the tetrahedral layer and interlayer K+ form a feldspar-like phase similar to leucite (KAISi2O6), the remainder forming a spinel, which, contrary to previous suggestions, appears to contain little silicon. Further heating induces the formation of mullite (AI6Si2OP13), and, in the higher-iron sample, corundum (α-Al2O3), in addition to the feldspar-like phase. The presence of the iron impurity enhances the recrystallization reactions and promotes the conversion of mullite to corundum, which eventually becomes the sole aluminous product in the high-iron sample. In samples fired to higher temperatures, only the tetrahedral aluminium resonance is detectable by27AI NMR, probably because most of the iron is located in either the mullite or corundum phases, in which it broadens the octahedral aluminium resonance beyond detection.
Similar content being viewed by others
References
N. H. Brett, K. J. D. Mackenzie andJ. H. Sharp,Quart. Revs. Chem. Soc. 24 (1970) 185.
J. P. Eberhart,Bull. Soc. Franc. Mineral. Crist. 86 (1963) 213.
S. Udagawa, K. Urabe andH. Hasu,Ganseki Kobutsu Kosho Gakkaishi 69 (1974) 381.
A. W. Nicol, “Clays and Clay Minerals”, in Proceedings of the 12th National Conference on Clays and Clay Minerals, Atlanta, 1963, edited by W. F. Bradley (Pergamon, Oxford, 1964) p. 11.
N. Sundius andA. M. Bystrom,Trans. Brit. Ceram. Soc. 52 (1953) 632.
G. W. Brindley, in “Progress in Ceramic Science, Vol. 3”, edited by J. E. Burke (Pergamon, Oxford, 1963) p. 1.
K. J. D. Mackenzie, I. W. M. Brown, R. H. Meinhold andM. E. Bowden,J. Amer. Ceram. Soc. 68 (1985) 266.
I. W. M. Brown, K. J. D. Mackenzie andR. H. Meinhold,J. Mater. Sci.
R. C. Mackenzie (ed) in “The Differential Thermal Investigation of Clays” (Mineralogical Society Monograph, London, 1957) Ch. 6.
W. E. Cameron,Bull. Am. Ceram. Soc. 56 (1977) 1003.
A. Muan andC. L. Gee,J. Amer. Ceram. Soc. 39 (1956) 207.
I. W. M. Brown, K. J. D. Mackenzie, M. E. Bowden andR. H. Meinhold,ibid. 68 (1985) 298.
J. Sanz andJ. M. Serratosa,J. Amer. Chem. Soc.,106 (1984) 4790.
E. Lippmaa, M. Magi, A. Samosan, G. Engelhardt andA. R. Grimmer,ibid. 102 (1980) 4889.
R. A. Kinsey, R. J. Kirkpatrick, J. Hower, K. A. Smith andE. Oldfield,Amer. Mineral. 70 (1985) 537.
C. P. Herrero, J. Sanz andJ. M. Serratosa,J. Phys. C. Solid State Phys. 18 (1985) 13.
R. J. Kirkpatrick, R. A. Kinsey, K. A. Smith, D. M. Henderson andE. Oldfield,Amer. Mineral. 70 (1985) 106.
B. L. Sherriff andJ. S. Hartman,Can. Mineral. 23 (1985) 205.
E. W. Radoslovich,Acta Crystallogr. 13 (1960) 919.
N. Güven,Z. Krist. 134 (1971) 196.
S. M. Richardson andJ. W. Richardson,Amer. Mineral. 67 (1982) 69.
S. Motherwell, “PLUTO”, A Programme for Plotting Molecular and Crystal Structures” (University Chemical Library, Cambridge, England, 1976).
J. Sanz andJ. M. Serratosa,Clay Mineral. 19 (1984) 113.
P. J. Malden andR. E. Meads,Nature 215 (1967) 844.
L. H. Brwen, S. B. Weed andJ. G. Stevens,Amer. Mineral. 54 (1969) 72.
C. S. Hogg andR. E. Meads,Mineralog. Mag. 37 (1970) 606.
B. A. Goodman,Mineralog. Mag. 40 (1976) 513.
H. Annersten andU. Halenius,Amer. Mineral. 61 (1976) 1045.
T. Ericsson, R. Wappling andK. Punakivi,Geol. Foeren. Stockholm Foerh.,99 (1977) 229.
J. Finch, A. R. Gainsford andW. C. Tennant,Amer. Mineral. 67 (1982) 59.
C. M. Cardile, I. W. M. Brown andK. J. D. Mackenzie, submitted toJ. Mater. Sci. Lett. 22 (1987) 357.
I. W. M. Brown, K. J. D. Mackenzie andC. M. Cardile,J. Mater. Sci. Lett. 22 (1987) 535.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mackenzie, K.J.D., Brown, I.W.M., Cardile, C.M. et al. The thermal reactions of muscovite studied by high-resolution solid-state 29-Si and 27-AI NMR. J Mater Sci 22, 2645–2654 (1987). https://doi.org/10.1007/BF01082158
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01082158