Abstract
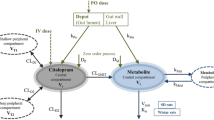
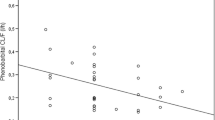
This investigation developed strategies along which the anticonvulsant effect of oxazepam in the rat could be pharmacokinetically modeled. After determination of the pharmacokinetics of oxazepam, which could be described with a two-compartment model (halflives of distribution and elimination 6 and 52 min, respectively), the drug was administered iv to groups of animals to achieve a serum concentration range of 0.1–2.5 mg/L at 10, 45, and 120 min after administration. At these time points pentylenetetrazol (PTZ) was infused slowly until the first myoclonic jerk occurred. The anticonvulsant response, expressed as the elevation of the serum or brain threshold concentration of PTZ, was modeled versus the serum (both total and free) and brain oxazepam concentration, according to the sigmoid E max model. The total serum and brain oxazepam EC50 values are about 0.5 mg/L and 1.1 mg/kg, respectively, and E max 120 mg/L PTZ. No marked differences in pharmacodynamic parameters between the three time groups were found, which indicates that serum and brain are pharmacokinetically indistinguishable from the effect compartment, that there is no (inter) activity of oxazepam metabolites and absence of development of acute tolerance during the investigated time frame. An interfering role of metabolites was also excluded by a direct radioreceptor assay of oxazepam, yielding very similar results as the specific Chromatographic assay. It is concluded that the concentration-anticonvulsant effect relationship of oxazepam can satisfactorily be described by the sigmoid E max model, when utilizing the employed experimental strategies.
Similar content being viewed by others
References
G. Levy. Pharmacokinetic and pharmacodynamic considerations in therapeutic drug concentration monitoring. In D. D. Breimer and P. Speiser (eds.),Topics in Pharmaceutical Sciences, Elsevier, Amsterdam, 1983, pp. 43–50.
J. Dingemanse, M. Danhof, and D. D. Breimer. Pharmacokinetic-pharmacodynamic modelling of CNS drug effects. An overview.Pharmacol. Ther. (in the press.)
M. Danhof and G. Levy. Kinetics of drug action in disease states. I. Effect of infusion rate on phenobarbital concentrations in serum, brain and cerebrospinal fluid of normal rats at onset of loss of righting reflex.J. Pharmacol. Exp. Ther. 229:44–50 (1984).
J. Dingemanse, D. Thomassen B. H. Mentink, and M. Danhof. Strategy to assess the role of (inter)active metabolites in pharmacodynamic studiesin vivo: A model study with heptabarbital.J. Pharm. Pharmacol. (in press).
M. Hisaoka, M. Danhof, and G. Levy. Kinetics of drug action in disease states. VII. Effect of experimental renal dysfunction on the pharmacodynamics of ethanol in rats.J. Pharmacol Exp. Ther. 232:717–721 (1985).
I. M. Ramzan and G. Levy. Kinetics of drug action in disease states. XIV. Effect of infusion rate on pentylenetetrazol concentrations in serum, brain and cerebrospinal fluid of rats at onset of convulsions.J. Pharmacol Exp. Ther. 234:624–628 (1985).
I. M. Ramzan and G. Levy. Kinetics of drug action in disease states. XVI. Pharmacodynamics of theophylline-induced seizures in rats.J. Pharmacol Exp. Ther. 236:708–713 (1986).
E. A. Swinyard and J. H. Woodhead. Experimental detection, quantification, and evaluation of anticonvulsants. In D. M. Woodbury, J. K. Penry, and C. E. Pippenger (eds.),Antiepileptic Drugs, Raven Press, New York, 1982, pp. 111–126.
W. Haefely, L. Pieri, P. Polc, and R. Schaffner. General pharmacology and neuropharmacology of benzodiazepine derivatives. In F. Hoffmeister and G. Stille (eds.),Handbook of Experimental Pharmacology, Vol. 55/II, Springer, Berlin, 1981, pp. 13–262.
C. Bellantuono, V. Reggi, G. Tognoni, and S. Garattini. Benzodiazepines: Clinical pharmacology and therapeutic use.Drugs 19:195–219 (1980).
J. R. Weeks and J. D. Davis. Chronic intravenous cannulas for rats.J. Appl. Physiol. 19:540–541 (1964).
D. E. Woolley and P. S. Timiras. Estrous and circadian periodicity and electroshock convulsions in rats.Am. J. Physiol. 202:379–382 (1962).
M. J. Orloff, H. L. Williams, and C. C. Pfeiffer. Timed intravenous infusion of metrazol and strychnine for testing anticonvulsant drugs.Proc. Soc. Exp. Biol Med. 70:254–257 (1949).
R. C. Chou and G. Levy. Effect of heparin or salicylate infusion on serum protein binding and on concentrations of phenytoin in serum, brain and cerebrospinal fluid of rats.J. Pharmacol. Exp. Ther. 219:42–48 (1981).
D. W. Esplin and D. M. Woodbury. The fate and excretion of C14-labeled pentylenetetrazol in the rat, with comments on analytical methods for pentylenetetrazol.J. Pharmacol. Exp. Ther. 118:129–138 (1956).
O. H. Lowry, H. J. Rosebrough, A. L. Farr, and R. J. Randall. Protein measurement with the Folin phenol reagent.J. Biol. Chem. 193:265–275 (1951).
R. J. Francis. ELSMOS-An extended least-squares modelling system in FORTRAN IV for mini- or micro-computer implementation.Comp. Prog. Biomed. 18:43–50 (1984).
S. Bolton.Pharmaceutical Statistics, Marcel Dekker, New York, 1985.
N. H. G. Holford and L. B. Sheiner. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models.Clin. Pharmacokin. 6:429–453 (1981).
E. A. Van der Velde. Orthogonal regression equation. In A. M. H. P. Van den Besselaar, H. R. Gralnick, and S. M. Lewis (eds.),Thromboplastin Calibration and Oral Anticoagulant Control, Martinus Nijhoff, The Hague, 1984, pp. 25–39.
P. L. Morselli, G. B. Cassano, G. F. Placidi, G. B. Muscettola, and M. Rizzo. Kinetics of the distribution of14C-diazepam and its metabolites in various areas of cat brain. In S. Garattini, E. Mussini, and L. O. Randall (eds.),The Benzodiazepines, Raven Press, New York, 1973, pp. 129–143.
S. Garattini. Active drug metabolites. An overview of their relevance in clinical pharmacokinetics.Clin. Pharmacokin. 10:216–227 (1985).
E. H. Ellinwood, M. Linnoila, M. E. Easier, and D. W. Molter. Profile of acute tolerance to three sedative anxiolytics.Psychopharmacology 79:137–141 (1983).
D. J. Nutt, P. J. Cowen. and A. R. Green. On the measurement in rats of the convulsant effect of drugs and the changes which follow electroconvulsive shock.Neuropharmacology 19:1017–1023 (1980).
D. J. Nutt, S. C. Taylor, and H. J. Little. Optimizing the pentetrazol infusion test for seizure threshold measurement.J. Pharm. Pharmacol. 38:697–698 (1986).
L. A. Woodbury and V. D. Davenport. Design and use of a new electroshock seizure apparatus, and analysis of factors altering seizure threshold pattern.Arch. Int. Pharmacodyn. Ther. 92:97–107 (1952).
F. Marcucci, M. L. Airoldi, E. Mussini, and S. Garattini. Brain levels of metrazol determined with a new gas chromatographic procedure.Eur. J. Pharmacol. 16:219–221 (1971).
W. D. Yonekawa, H. J. Kupferberg, and D. M. Woodbury. Relationship between pentylenetetrazol-induced seizures and brain pentylenetetrazol levels in mice.J. Pharmacol. Exp. Ther. 214:589–593 (1980).
S. Garattini, E. Mussini, F. Marcucci, and A. Guaitani. Metabolic studies on benzodiazepines in various animal species. In S. Garattini, E. Mussini, and L. O. Randall (eds.),The Benzodiazepines, Raven Press, New York, 1973, pp. 75–97.
P. T.-H. Wong, Y. L. Yoong, and M. C. E. Gwee. Acute tolerance to diazepam induced by benzodiazepines.Clin. Exp. Pharmacol. Physiol. 13:1-8 (1986).
D. P. Crankshaw and C. Raper. The effect of solvents on the potency of chlordiazepoxide, diazepam, medazepam and nitrazepam.J. Pharm. Pharmacol. 23:313–321 (1971).
K. J. Davis and P. M. Jenner. Toxicity of three drug solvents.Toxicol. Appl. Pharmacol 1:576–578 (1959).
A. Kutzsche. Zur Toxikologie des Dimethylformamids.Arzneim. Forsch. 15:618–624 (1965).
F. Marcucci, E. Mussini, R. Fanelli, and S. Garattini. Species differences in diazepam metabolism-I. Metabolism of diazepam metabolites.Biochem. Pharmacol. 19:1847–1851 (1970).
J. R. M. Haigh, J. P. Gent, and R. Calvert. Plasma concentrations of clobazam and its N-desmethyl metabolite; protection against pentetrazol-induced convulsions in mice.J. Pharm. Pharmacol. 36:636–638 (1984).
S. M. Taylor, G. D. Bennett, L. C. Abbott, and R. H. Finnell. Seizure control following administration of anticonvulsant drugs in the quaking mouse.Eur. J. Pharmacol. 118:163–170 (1985).
G. M. Pollack and D. D. Shen. A timed intravenous pentylenetetrazol infusion seizure model for quantitating the anticonvulsant effect of valproic acid in the rat.J. Pharmacol. Methods 13:135–146 (1985).
R. M. Arendt, D. J. Greenblatt, R. H. DeJong, J. D. Bonin, D. R. Abernethy, B. L. Ehrenberg, H. G. Giles, E. M. Sellers, and R. I. Shader.In vitro correlates of benzodiazepine cerebrospinal fluid uptake, pharmacodynamic action and peripheral distribution.J. Pharmacol. Exp. Ther. 227:98–106 (1983).
C. Harriton, L. Ciesielski, S. Simler, M. Valli, G. Jadot, S. Gobaille, E. Mesdjian, and P. Mandel. Distribution of sodium valproate and GABA metabolism in CNS of the rat.Biopharm. Drug Disp. 5:409–414 (1984).
R. P. Simon, N. L. Benowitz, J. Bronstein, J., and P. Jacob. Increased brain uptake of lidocaine during bicuculline-induced status epilepticus in rats.Neurology 32:196–199 (1982).
R. P. Simon, N. L. Benowitz, and S. Culala. Motor paralysis increases brain uptake of lidocaine during status epilepticus.Neurology 34:384–387 (1984).
R. P. Simon, N. Benowitz, R. Hedlund, and J. Copeland. Influence of the blood-brainpH gradient on brain phenobarbital uptake during status epilepticus.J. Pharmacol. Exp. Ther. 234:830–835 (1985).
M. Clozel, J. L. Daval, P. Monin, C. Dubruc, P. L. Morselli, and P. Vert. Regional cerebral blood flow during bicuculline-induced seizures in the newborn piglet: effect of phenobarbital.Dev. Pharmacol. Ther. 8:189–199 (1985).
B. Oztas and U. Sandalci. Blood-brain barrier permeability after pentylenetetrazol and electrically induced seizure. IRCS (Int. Res. Commun. Syst.)Med. Sci. Biochem. 12:488–489 (1984).
E. M. Sellers, C. A. Naranjo, V. Khouw, and D. J. Greenblatt. Binding of benzodiazepines to plasma proteins. In E. Usdin, P. Skolnick, J. F. Tallman, D. Greenblatt, and S. M. Paul (eds.),Pharmacology of Benzodiazepines, Macmillan, London, 1982, pp. 271–284.
S. F. Sisenwine, C. O. Tio, S. R. Shrader, and H. W. Ruelius. The biotransformation of oxazepam in man, miniature swine and rat.Arzneim. Forsch. 22:682–687 (1972).
S. F. Sisenwine and C. O. Tio. The metabolic disposition of oxazepam in rats.Drug Metab. Dispos. 14:41–45 (1986).
D. J. Greenblatt. Clinical pharmacokinetics of oxazepam and lorazepamClin. Pharmacokin. 6:89–105 (1981).
L. Aaltonen and M. Scheinin. Application of radioreceptor assay of benzodiazepines for toxicology.Acta Pharmacol. Toxicol. 50:206–212 (1982).
D. B. Barnett and S. R. Nahorski. Current techniques: drug assays in plasma by radio- receptor techniques.Trends Pharmacol. Sci. 4:407–409 (1983).
M. K. Ticku. Convulsant binding sites on the benzodiazepine/GABA receptor. In R. W. Lsen and J. C. Venter (eds.),Benzodiazepine/GABA Receptors and Chloride Channels. Structural and Functional Properties, Alan R. Liss, New York, 1986, pp. 195–207.
R. Ramanjaneyulu and M. K. Ticku. Interactions of pentamethylenetetrazole and tetrazole analogues with the picrotoxinin site of the benzodiazepine-GABA receptor-ionophore complex.Eur. J. Pharmacol. 98:337–345 (1984).
M. Rehavi, P. Skolnick, and S. M. Paul. Effects of tetrazole derivatives on [3H]diazepam bindingin vitro: correlation with convulsant potency.Eur. J. Pharmacol. 78:353–356 (1982).
G. Blaschke and H. Markgraf. Chromatographische Racemattrennungen, IX. Chlortalidon-, Chlortalidon-methylether und Oxazepam-Enantiomere.Chem. Ber. 113:2031–2035 (1980).
J. L. Waddington and F. Owen. Stereospecific benzodiazepine receptor binding by the enantiomers of oxazepam sodium hemisuccinate.Neuropharmacology 17:215–216 (1978).
H. Kalant, A. E. LeBlanc, and R. J. Gibbins. Tolerance to, and dependence on, some non-opiate psychotropic drugs.Pharmacol. Rev. 23:135–191 (1971).
Y. L. Yoong, H. S. Lee, M. C. E. Gwee, and P. T.-H. Wong. Acute tolerance to diazepam in mice: pharmacokinetic considerationsClin. Exp. Pharmacol. Physiol. 13:153–158 (1986).
R. G. Lister and D. J. Nutt. Mice and rats are sensitized to the proconvulsant action of a benzodiazepine-receptor inverse agonist (FG7142) following a single dose of lorazepam.Brain Res. 379:364–366 (1986).
M. I. Gluckman and B. L. Baxter. Comparative effects of single and repeat dose administration of minor tranquilizers.Psychopharmacol. Bull. 7:26–27 (1971).
S. A. Henauer, E. J. Gallaher, and L. O. Hollister. Long-lasting single-dose tolerance to neurologic deficits induced by diazepam.Psychopharmacology 82:161–163 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dingemanse, J., Sollie, F.A.E., Breimer, D.D. et al. Pharmacokinetic modeling of the anticonvulsant response of oxazepam in rats using the pentylenetetrazol threshold concentration as pharmacodynamic measure. Journal of Pharmacokinetics and Biopharmaceutics 16, 203–228 (1988). https://doi.org/10.1007/BF01062261
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01062261