Abstract
A survey is given of theoretical asymptotic bubble behaviour which is governed by heat or/and mass diffusion towards the bubble boundary. A model has been developed to describe the effect of turbulent forced flow on both bubble behaviour and ohmic resistance. A comparison with experimental results is also made.
Similar content being viewed by others
Abbreviations
- ga :
-
liquid thermal diffusivity (m2 s−1)
- B :
-
width of electrode (m)
- c :
-
liquid specific heat at constant pressure (J kg−1 K−1)
- ΔC 0 :
-
initial supersaturation of dissolved gas at the bubble wall (kg m−3)
- d :
-
bubble density at electrode surface (m−2)
- D :
-
diffusion coefficient of dissolved gas (m2 s−1)
- D h :
-
−4S/Z, hydraulic diameter, withS being the cross-sectional area of the flow andZ being the wetted perimeter (m)
- e :
-
base of natural logarithms, 2.718...
- f :
-
local gas fraction
- F :
-
Faraday constant (C kmol−1)
- G :
-
evaporated mass diffusion fraction
- h :
-
height from bottom of the electrode (m)
- h w :
-
total heat transfer coefficient for electrode surface (J s−1 m−2 K−1)
- h w,conv :
-
convective heat transfer coefficient for electrode surface (J s−1 m−2K−1)
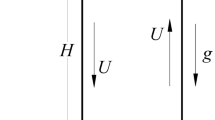
- H :
-
total height of electrode (m)
- i :
-
electric current density (A m−2)
- j, j * :
-
number
- Jα :
-
modified Jakob number,ΔC 0/ρ 2
- ℒ :
-
enthalpy of evaportion (J kg−1)
- m :
-
density of activated nuclei generating bubbles at electrode surface (m−2)
- n :
-
product of valency and number of equal ions forming one molecule; for hydrogenn=2, for oxygenn=4
- p :
-
pressure (N m−2)
- Δp :
-
excess pressure (N m−2)
- R :
-
gas constant (J kmol−1 K−1)
- R 1 :
-
bubble departure radius (m)
- R 0 :
-
equilibrium bubble radius (m)
- ΔR/R :
-
relative increase of ohmic resistance due to bubbles, ΔR, in comparison to corresponding value,R, for pure electrolyte
- Re :
-
Reynolds number,νD h/ν
- Sc :
-
Schmidt number,ν/D
- Sh :
-
Sherwood number\({{D_h } \mathord{\left/ {\vphantom {{D_h } {\bar \delta _m }}} \right. \kern-\nulldelimiterspace} {\bar \delta _m }}\)
- t :
-
time (s)
- T :
-
absolute temperature (K)
- ΔT :
-
increase in temperature of liquid at bubble boundary with respect to original liquid in binary mixture (K)
- gu :
-
solution flow velocity (m s−1)
- x :
-
mass fraction of more volatile component in liquid at bubble boundary in binary mixture
- x 0 :
-
mass fraction of more volatile component in original liquid in binary mixture
- y :
-
mass fraction of more volatile component in vapour of binary mixture
- α :
-
contact angle
- δ:
-
local thickness of one phase velocity boundary layer (m)
- δm :
-
local thickness of corresponding mass diffusion layer (m)
- δ* :
-
local thickness of two-phase velocity boundary layer (m)
- θ o :
-
initial liquid superheating (K)
- κ :
-
constant in Henry's law (m2 s−2)
- ν :
-
liquid kinematic viscosity (m2 s−1)
- ν * :
-
bubble frequency at nucleus (s−1)
- ρ 1 :
-
liquid mass density (kg m−3)
- ϱ 2 :
-
gas/vapour mass density (kg m−3)
- σ:
-
surface tension (N m−1)
References
[1]S. J. D. van Stralen, R. Cole, ‘Boiling Phenomena’, Vols. 1 and 2, Hemisphere, Washington/McGraw-Hill, New York (1979).
L. J. J. Janssenand S. J. D. van Stralen,Electrochim. Acta 26 (1981) 1011.
P. C. Slooten, PhD Thesis, Eindhoven University of Technology, Eindhoven (1984).
H. M. S. Wedershoven, R. M. de Jonge, C. W. M. P. Sillen and S. J. D. van Stralen,Int J. Heat Mass Transfer 25 (1982) 1239.
B. E. Bongenaar-Schlenter, PhD Thesis, Eindhoven University of Technology, Eindhoven (1984).
B. E. Bongenaar-Schlenter, L. J. J. Janssen, S. J. D. van Stralen and E. Barendrecht,J. Appl. Chem. 15 (1985) 537.
C. W. M. P. Sillen, PhD Thesis, Eindhoven University of Technology, Eindhoven (1983).
P. J. M. Thommassen, M. Thesis, Eindhoven University of Technology, Eindhoven (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Stralen, S.J.D., Sluyter, W.M. Mass diffusion-controlled bubble behaviour in boiling and electrolysis and effect of bubbles on ohmic resistance. J Appl Electrochem 15, 527–536 (1985). https://doi.org/10.1007/BF01059294
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01059294