Abstract
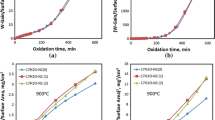
This paper describes the oxidation behavior of low-carbon steel samples in binary gas mixtures of oxygen and nitrogen, at oxygen concentrations ranging between 1% and 15% and temperatures ranging between 1000 and 1250°C. Sample weight gains versus time were analyzed, along with measurements and calculations of sample heating rates due to exothermic heat of reaction at the sample surface. It was found that initial rates of oxidation depended on oxygen content in the gasmixture and that these reaction rates were linear up to oxide thicknesses of 0.4 to 0.5 mm. Calculations of linear oxidation rate constants based on equations for mass transport of oxygen in the gas mixture to the sample surface showed good agreement with those measured experimentally, indicating that the initial period of oxidation is controlled by the mass transport of oxygen to the reaction interface. The linear rate constants showed little dependency on temperature, an activation energy of approximately 17kJ/mole being obtained. Measurements of sample surface temperatures have shown that within this linear-oxidation regime, interfacial temperatures of the samples increase with increasing oxygen contents in the gas mixture, owing to exothermic heats of oxidation. Subsequent oxidation kinetics were found to be parabolic. Measured parabolic rates constants were in good agreement with previous investigations, with activation energy values of approximately 127kJ/mole.
Similar content being viewed by others
References
Chengyu Yan and Franz Oeters,Steel Res. 65, 355–361 (1994).
R. Longani and W. W. Smeltzer,Oxid. Met. 1, 3–21 (1969).
Von H.-J. Grabke,Ber. Bunsenge. 69, 48–57 (1965).
F. S. Pettit and J. B. Wagner, Jr.,Acta Metall. 12, 35–40 (1964).
F. Pettit, R. Yinger, and J. B. Wagner, Jr.,Acta Metall. 8, 617–623 (1960).
W. W. Smeltzer,Acta Metall. 8, 377–383 (1960).
L. A. Morris and W. W. Smeltzer,Acta Metall. 15, 1591–1596 (1967).
H. Abuluwefa, R. I. L. Guthrie, and F. Mucciardi, Steel Reheat Furnace Proceedings (CIM, The Iron and Steel Society of AIME, 1992), pp. 243–267.
C. Wagner,Z. Phys. Chem. B21, 25 (1933).
L. Himmel, R. F. Mehl, and C. E. Birchenall,J. Met. Trans. AIME June, 827–843 (1953).
M. H. Davies, M. T. Simnad, and C. E. Birchenall,J. Met. Trans. AIME October, 889–896 (1951).
Per Kofstad,High Temperature Corrosion (University of Oslo, Norway, 1988).
R. C. Longani and W. W. Smeltzer,Can. Metall. Q. 10, 149–163 (1971).
Per Kofstad and S. Espevik,J. Electrochem. Soc. 112, 153–160 (1965).
Hans-Joachim Selenz and Franz Oeters, Report from the Institute of Metallurgy (Ferrous Metallurgy) of Berlin Technical University; the Publication is part of Dr.-Ing thesis, 1984.
Von A. Rahmel,Werks. Korros. 23, 95–98 (1972).
Von J. Deich and F. Oeters,Werks. Korros. 24, 365–371 (1973).
G. J. Yurek, J. P. Hirth, and R. A. Rapp,Oxid. Met. 8, 265–281 (1974).
G. Garnaud and R. Rapp,Oxid. Met. 11, 193–198 (1977).
V. G. Levich,Physicochemical Hydrodynamics, (Prentice Hall, Englewood Cliffs, NJ, 1962), pp. 87–91.
S. Chapman and T. G. Cowling,Mathematical Theory of Non-Uniform Gases, 3rd ed. (Cambridge University Press, 1970).
R. B. Rird, W. E. Stewart, and E. N. Lightfoot,Transport Phenomena (Wiley, New York, 1960).
H. Abuluwefa, T. H. Root, R. I. L. Guthrie, and F. Ajersch,Metall. Trans. B, to appear.
J. P. Holman,Heat Transfer, 6th ed. (McGraw-Hill, 1963), pp. 225–231.
K. Sachs and C. W. Tuck,Werks. Korros 21, 945–954 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abuluwefa, H., Guthrie, R.I.L. & Ajersch, F. The effect of oxygen concentration on the oxidation of low-carbon steel in the temperature range 1000 to 1250°C. Oxid Met 46, 423–440 (1996). https://doi.org/10.1007/BF01048639
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01048639