Summary
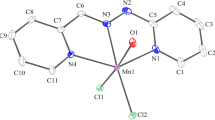
The three new potential chelating ligands dihydridobis-, hydridotris- and tetrakis-(thiophenolyl)borate anions, and their chelates with first row transition metals have been synthesised. The divalent and trivalent metal ions form complexes in 1∶2 and 1∶3 (metal:ligand) ratios respectively. The number of ligands coordinated correspond to the number of anions replaced in the metal salis. The compounds were characterized by elemental analysis, i.r. spectra, magnetic susceptibility measurements and electronic spectral studies. The CrIII and FeIII complexes of dihydridobis- and hydridotris-(thiophenolyl)borates appear to be octahedral, and those of CuII are proposed to be square planar. Tetrahedral geometry is suggested for the MnII, CoII and NiII complexes. The tetrakis-(thiophenolyl)borate yielded octahedral complexes with all the metal ions except for CuII which is square planar. The ligand field parameters 10Dq, B and β have also been calculated wherever possible. The ligands may be placed in the vicinity of EDTA in the nephelauxetic series.
Similar content being viewed by others
References
S. Trofimenko,J. Am. Chem. Soc. 88, 1842 (1966);89, 3158, 3165, 3170 (1967);Chem. Rev.,72, 497 (1972).
M. K. Das, K. Niedenzu and H. Nöth,Inorg. Chem.,27, 1112 (1988).
C. P. Brock, K. Niedenzu, E. Hanecker and H. Nöth,Acta Crystallogr. Sect C., Cryst Struct. Commun.,C41, 1458 (1985).
G. Socrates,Infrared Characteristic Group Frequencies, Wiley, 1980, p. 33, 34.
A. S. Gates and J. G. Edwards,Inorg. Chem.,16, 2252 (1977).
J. W. Nibler, D. F. Shriver and T. H. Cook,J. Chem. Phys.,54, 5257 (1971).
L. J. Bellamy,The Infrared spectra of complex Molecules, Methuen, 1956, p. 356.
K. Nakamoto,Infrared spectra of Inorganic and Coordination Compounds, John Wiley and Sons, New York, 1963, p. 214.
B. N. Figgis,Introduction to Ligand Fields, Wiley Eastern Ltd., New Delhi, 1966, Ch. 9.
B. N. Figgis and J. Lewis in J. Lewis and R. G. Wilkins (Eds.),Modern Coordination Chemistry, Interscience, New York, 1960, Ch. 6.
Z. A. Siddiqi, S. Khan, K. S. Siddiqi and S. A. A. Zaidi,Synth. React. Inorg. Met. Org. Chem.,14, 303 (1984).
S. J. Barclay and K. N. Raymond,Inorg. Chem.,25, 3561 (1986).
R. L. Carlin,Transition Met. Chem., Marcel Dekker, New York, Vol. 1, 1965.
S. Chandra and K. K. Sharma,Synth. React. Inorg. Met.-Org. Chem.,13, 570 (1983).
R. D. Willet, O. L. Liles Jr. and C. Michelson,J. Inorg. Chem., 6 (1967).
L. E. Orgel,J. Chem. Phys.,23, 1004 (1955).
J. P. Jesson, S. Trofimenko and D. R. Eaton,J. Am. Chem. Soc.,89, 3158 (1967).
A. I. Vogel,Textbook of Quantitative Inorganic Analysis, Longmans Green and Co, 1986, pp. 505–507.
C. N. Reilly, R. W. Schmid and F. S. Sadek,Chem. Educ.,36, 555, 619 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zaidi, S.A.A., Zahoor, M.A. & Siddiqi, K.S. Transition metal complexes of potassium dihydridobis-, hydridotris- and tetrakis-(thiophenolyl)borate anions. Transition Met Chem 15, 231–235 (1990). https://doi.org/10.1007/BF01038381
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01038381