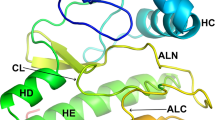
Abstract
Comparisons of nine creatine kinase sequences show that 67% of the protein sequence is identical among rabbit, rat, mouse, and chicken muscle, rabbit, rat, and chicken brain, and electric organ sequences from two species of electric ray(Torpedo). The extensive homology precludes a facile prediction of active-site residues based on sequence conservation. The sequences are more similar within isozyme types than are the different isozymes from any one species. There are 35 positions in the muscle and brain sequence pairs for three species which differentiate the two forms. TheTorpedo sequences do not fall completely into either of these patterns. Except for homology with partial sequences of other ATP-guanidino phosphotransferases, no significant homology with other protein or nucleic acid sequences in available databases was found. Preliminary secondary structural predictions suggest that the C-terminal half of the protein is likely an α/β-type protein. Placement in the sequence of two peptides found in previous cross-linking studies reveals two stretches of primary structure that are presumably close in space to the reactive Cys-283 and hence close to the active site.
Similar content being viewed by others
References
Abarbanel, R. M. (1985). Ph.D. Thesis, University of California, San Francisco, California.
Barker, W. C., Hunt, L. T., Orcutt, B. C., George, D. G., Yeh, L. S., Chem, H. R., Blomquist, M. C., Johnson, G. C., Seibel-Ross, E. I., and Ledley, R. S. (1984a). Protein Identification Resource of the NIH, National Biomedical Research Foundation, Georgetown University Medical Center, Washington, D.C.
Barker, W. C., Chen, H. R., Hunt, L. T., Orcutt, G. C., Seibel-Ross, E. I., and Dayhoff, M. O. (1984b). Nucleic Acid Sequence Database, Version 22, National Biomedical Research Foundation, Georgetown University Medical Center, Washington, D.C.
Barrantes, F. J., Mieskes, G., and Walliman, T. (1983).FEBS Lett. 152, 270–276.
Benfield, P. A., Ziven, R. A., Miller, L. S., Sowder, R., Smythers, G. W., Henderson, L., Oroszlan, S., and Pearson, M. L. (1984).J. Biol. Chem. 259, 14979–14984.
Benfield, P. A., Henderson, L., and Pearson, M. L. (1985).Gene 39, 263–267.
Buskin, J. N., Jaynes, J. B., Chamberlain, J. S., and Hauschka, S. D. (1985).J. Mol. Evol.,22, 334–341.
Caravatti, M., Perriard, J. C., and Eppenberger, H. M. (1979).J. Biol. Chem. 254, 1388–1394.
Cohen, F. E., Abarbanel, R. M., Kuntz, I. D., and Fletterick, R. J. (1983).Biochemistry 22, 4894–4904.
Cohen, F. E., Abarbanel, R. M., Kuntz, I. D. and Fletterick, R. J. (1986).Biochemistry,25, 266–275.
Cohn, M., and Reuben, J. (1971).Acc. Chem. Res. 4, 214–219.
Dawson, D. M., Eppenberger, H. M., and Kaplan, N. O. (1967).J. Biol. Chem. 242, 210–217.
DeLuca, M., Hall, N., Rice, R., and Kaplan, N. D. (1981).Biochem., Biophys. Res. Comm. 99, 189–195.
DerTerrosian, E., Pradel, L. A., Kassab, R., and van Thoai, N. (1969).Eur. J. Biochem. 11, 482–490.
DerTerrosian, E., Desvages, G., Pradel, L. A., Kassab, R., and van Thoai, N. (1971).Eur. J. Biochem. 22, 585–592.
Dickerson, R. E. (1971).J. Mol. Evol. 1, 26–45.
Doolittle, R. F. (1981).Science 214, 149–159.
Doolittle, R. F. (1983).Newat83, Department of Chemistry, University of California, San Diego, California.
Eppenberger, H. M., Dawson, D. M., and Kaplan, N. D. (1967).J. Biol. Chem. 242, 204–209.
Eppenberger, H. M., Perriard, J.-C., and Walliman, T., 1983, InIsozymes: Current Topics in Biological and Medical Research, Vol. 7 (Rattozzi, M., Scandilios, J. C., and Whitt, G. S., eds.), Alan R. Liss, New York, pp. 19–38.
Fisher, M. L., Carliner, N. H., Becker, L. C., Peters, R. W., and Plotnick, G. D. (1983).J. Am. Med. Assoc. 249, 393–394.
GenBank (1984). Release 23.0, European Molecular Biology Laboratory.
Giraudat, J., Devillers-Thiery, A., Perriard, J.-C., and Changeux, J.-P. (1984).Proc. Natl. Acad. Sci. USA 81, 7313–7317.
Gysin, R., and Flanagan, S. D. (1985).Fed. Proc. 44, 2817.
Kenyon, G. L., and Reed, G. H. (1983).Adv. Enzymol. 54, 367–426.
Knell, J. D., Perryman, M. B., Bohlmeyer, T. J., and Roberts, R. (1985).Fed. Proc. 44, 1464.
Kwiatkowski, R. W., Schweinfest, C. W., and Dottin, R. P. (1984).Nucleic Acids Res. 12, 6925–6934.
Lipman, D. J., and Pearson, W. R. (1985a).Science 22, 1435–1441.
Lipman, D. J., and Pearson, W. R. (1985b).Proc. Natl. Acad. Sci. USA 80, 726–730.
Mahowald, T. A. (1969).Fed. Proc. 28, 601.
Martinez, H. M., and Sobel, E. (1985).Nucleic Acids Res., in press.
Martinez, H. M., Katzung, B., and Farrah, T. (1983).Sequence Analysis Program Manual, Biomathematics Computation Laboratory, University of California, San Francisco.
Orcutt, B. C., Dayhoff, M. O., and Barker, W. C. (1984). Protein Identification Resource, National Biomedical Research Foundation, Georgetown University Medical Center, Washington, D.C.
Ordahl, C. P., Evans, G. L., Cooper, T. A., Kunz, G., and Perriard, J.-C. (1984).J. Biol. Chem. 259, 15224–15227.
Perriard, J.-C. (1979).J. Biol. Chem. 254, 7036–7041.
Perriard, J.-C., Achtnich, U., Cerny, L. C., Eppenberger, H. M., Grove, B. K., Hossle, J. P., and Schäfer, B. (1985). InMolecular Biology of Muscle Development (Emerson, C., Fischman, D. A., Nadal-Ginand, B., and Siddigin, M. A. Q., eds.), Alan R. Liss, New York, in press.
Perryman, M. B., Knell, J. D., Ifegwu, J., and Roberts, R. (1985).J. Biol. Chem. 260, 9399–9404.
Pickering, L., Pang, H., Biemann, K., Monro, H., and Schimmel, P. (1985).Proc. Natl. Acad. Sci. USA 82, 2310–2314.
Putney, S., Herlihy, W., Royal, N., Pang, H. Y., Aposhian, H. V., Pickering, L., Belagage, R., Biemann, K., Page, D., Kuby, S., and Schimmel, P. (1984).J. Biol. Chem. 259, 14317–14320.
Regnouf, F., Kassals, R., Debuire, B., Richard, C., and Han, K. K. (1981).Int. J. Peptide Protein Res. 17, 143–155.
Reiss, N. A., and Kaye, A. M. (1980).J. Biol. Chem. 256, 5741–5749.
Richardson, J. S. (1981).Adv. Protein Chem. 34, 167–355.
Roman, D. G., Billadello, J. J., Gordon, J. I., Sobel, B. E., and Strauss, A. W. (1985).Clin. Res. 33, 222A.
Rosenberg, U. B., Kunz, G., Frischauf, A., Lehrach, H., Mahr, R., Eppenberger, H. M., and Perriard, J.-C. (1982).Proc. Natl. Acad. Sci. USA 79, 6589–6592.
Walliman, T., and Eppenberger, H. M. (1985). InCell and Muscle Motility, Vol. 6 (J. W. Shay, ed.), Plenum Press, New York, pp. 239–285.
Walliman, T., Walzthony, D., Wegmann, G., Moser, H., Eppenberger, H. M., and Barrantes, F. J. (1985).J. Cell Biol. 100, 1063–1072.
Watts, D. C. (1973). InThe Enzymes, Vol. 8, Part A (Boyer, P. D., ed.), Academic Press, New York, pp. 383–455.
West, B. L., Babbitt, P. C., Mendez, B., and Baxter, J. D. (1984).Proc. Natl. Acad. Sci. USA 81, 7007–7012.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Babbitt, P.C., Kenyon, G.L., Kuntz, I.D. et al. Comparisons of creatine kinase primary structures. J Protein Chem 5, 1–14 (1986). https://doi.org/10.1007/BF01025580
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01025580