Abstract
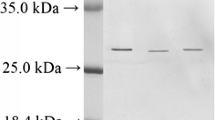
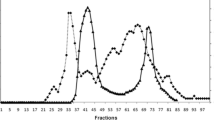
A basic trypsin-subtilisin inhibitor has been isolated from the egg white of marine turtle (Caretta caretta Linn.) and purified to homogeneity by gel filtration followed by ion-exchange chromatography. It has a single polypeptide chain of 117 amino acid residues, having a molecular weight of 13,600. It lacks methionine and tryptophan. Its isoelectric point is atpH 10.0 and the sedimentation coefficient (s20,w) value of 1.62 S is independent of protein concentration. It has a Stokes radius of 18.8 Å, an intrinsic viscosity of 0.048 dl g−1 and a diffusion coefficient of 10.17×10−7 cm2 sec−1. Its fluorescence emission spectrum is similar to that of free tyrosine and the bimolecular quencing rate constant of its tyrosine residues with acrylamide is 3.15×109 M−1 sec−1. The inhibitor strongly inhibits both trypsin and subtilisin by forming enzyme-inhibitor complexes at a molar ratio of unity. The nature of inhibition toward both enzymes is not temporary. It has independent binding sites for inhibition of trypsin and subtilisin. Chemical modification with tetranitromethane suggests the presence of three tyrosine residues on the surface of the inhibitor molecule.
Similar content being viewed by others
References
Baldwin, R. L. (1957).Biochem. J. 65, 490–502.
Birks, J. (1970).Photophysics of Aromatic Molecules, Wiley-Interscience, New York, pp. 433–447.
Bode, W., Epp, O., Huber, R., Laskowski, M., Jr., and Ardelt, W. (1985).Eur. J. Biochem. 147, 387–395.
Chase, T., and Shaw, E. (1967).Biochem. Biophys. Res. Commun. 29, 508–514.
Cohn, E. J., and Edsall, J. T. (1943).Proteins, Amino Acids and Peptides, Reinhold, New York, pp. 370–381.
Davis, B. J. (1964).Ann. NY Acad. Sci. 121, 404–427.
Ellman, G. L. (1959).Arch. Biochem. Biophys. 82, 70–77.
Green, N. M., and Work, E. (1953).Biochem. J. 54, 347–352.
Grutler, M. G., Fendrich, G., Huber, R., and Bode, W. (1988).EMBO J. 7, 345–351.
Guha, M. K., and Sinha, N. K. (1984).J. Biosci. 6, 155–163.
Habeeb, A. F. S. A. (1966).Anal. Biochem. 14, 328–336.
Hartley, B. S. (1970).Biochem. J. 119, 805–822.
Heinzel, R., Appelhans, H., Gassen, H.-G., Seemuller, U., Arnhold, M., Fritz, H., Lottspeich, F., Widenmann, K., and Machleidt, W. (1987).Pulmonary Emphysema and Proteolysis: 1986 (Taylor, J. C., and Mittman, C., eds.), Academic Press, New York, pp. 297–306.
Hirs, C. H. W. (1967).Methods Enzymol. 11, 197–199.
Kassell, B. (1970).Methods Enzymol. 19, 840–844.
Kato, I., and Tominaga, N. (1979).Fed. Proc. 38, 832.
Kato, I., Schrode, J., Kohr, W. J., and Laskowski, M., Jr. (1987).Biochemistry 26, 193–201.
Kragh, A. M. (1961).A Laboratory Manual of Analytical Methods of Protein Chemistry (Alexander, P., and Block, R. J., eds.), Pergamon, New York, Vol. 2, pp. 173–209.
Kramps, J. A., Van Twisk, C., Heribert, A., Barbara, M., Nikiforow, T., and Dijkman, J. H. (1990).Biochim. Biophys. Acta 1038, 178–185.
Lakowicz, J. R. (1983).Principles of Fluorescence Spectroscopy, Plenum Press, New York, Chapter 2, pp. 44–45.
Laskowski, M., Jr., and Sealock, R. W. (1971).The Enzymes (Boyer, P. D., ed.), Academic Press, New York, Vol. 3, pp. 375–473.
Laskowski, M., Jr., Kato, I., Leary, T. R., Schrode, J., and Sealock, R. W. (1974).Proteinase Inhibitors, 2nd Int. Res. Conf., Bayer Symp. V (Fritz, H., Tschesche, H., Greene, L. J., and Truscheit, E., eds.), Springer-Verlag, New York, pp. 597–611.
Laskowski, M., Jr., Kato, I., Ardelt, W., Cook, J., Denton, A., Empie, M. W., Kohr, W. J., Park, S. J., Parks, K., Schatzley, B. L., Schoenberger, O. L., Tashiro, M., Vichot, G., Whatley, H. E., Wieczorek, A., and Wieczorek, M. (1987).Biochemistry 26, 202–221.
Laskowski, M., Jr., Apostol, I., Ardelt, W., Cook, J., Giletto, A., Kelly, C. A., Lu, W., Park, S. J., Qasim, M. A., Whatley, H. E., Wieczorek, A., and Wgnn, R. (1990).J. Prot. Chem. 9, 715–725.
Lin, Y., and Feeney, R. E. (1972).Glycoproteins (Gottschalk, A., ed.), Elsevier, Amsterdam, Part B, pp. 762–782.
Liu, T. Y., and Chang, Y. H. (1971).J. Biol. Chem. 246, 2842–2848.
Liu, W. H., Feinstein, H. G., Osuga, D. T., Haynes, R., and Feeney, R. E. (1968).Biochemistry 7, 2886–2892.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951).J. Biol. Chem. 193, 265–275.
Matsubara, H., Kasper, C. B., Brown, D. M., and Smith, E. L. (1965).J. Biol. Chem. 240, 1125–1130.
Musil, D., Bode, W., Huber, R., Laskowski, M., Jr., Lin, T., and Ardelt, W. (1991).J. Mol. Biol. 220, 739–755.
Ozawa, K., and Laskowski, M., Jr. (1966).J. Biol. Chem. 241, 3955–3961.
Papamokos, E., Weber, E., Bode, W., Huber, R., Empie, M. W., Kato, I., and Laskowski, M., Jr. (1982).J. Mol. Biol. 158, 515–537.
Ray, A. K., Guha, M. K., and Sinha, N. K. (1982).Biochim. Biophys. Acta 716, 126–132.
Reisfeld, R. E., Lewis, U. J., and Williams, D. E. (1962).Nature (Lond.)195, 281–283.
Riordan, J. F., and Vallee, B. L. (1972).Methods Enzymol. 25B, 515–521.
Schachman, H. K. (1957).Methods Enzymol. 4, 32–103.
Siegel, L. M., and Monty, K. J. (1966).Biochim. Biophys. Acta 112, 346–362.
Spackman, D. H., Stein, W. H., and Moore, S. (1958).Anal. Chem. 30, 1190–1206.
Swank, R. T., and Munkres, K. D. (1971).Anal. Biochem. 39, 462–477.
Vesterberg, O., and Svenson, H. (1966).Acta Chem. Scand. 20, 820–834.
Walsch, K. A., and Wilcox, P. E. (1970).Methods Enzymol. 19, 31–41.
Weber, E., Papamokos, E., Bode, W., Hube, R., Kato, I., and Laskowski, M., Jr. (1981).J. Mol. Biol. 149, 109–123.
Whitaker, J. R. (1963).Anal. Chem. 35, 1950–1953.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sil, P.C., Chaudhuri, T.K. & Sinha, N.K. Basic trypsin-subtilisin inhibitor from marine turtle egg white: Hydrodynamic and inhibitory properties. J Protein Chem 12, 71–78 (1993). https://doi.org/10.1007/BF01024917
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01024917