Summary
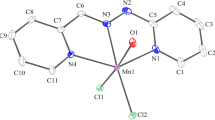
The ligand, potassium bicyclo[2.2.1]-hept-5-en-endo-2-oyl-hydroxylamine-3-carboxylate-monohydrate, KHL·H2O2 and its M(HL)2 complexes, [{Fe(HL)2}2SO4], K[FeL2] and K2[ML2] (M=MnII, FeII, CoII, NiII, CuII and ZnII) were prepared and characterized. For all, except the sulphate complex of iron(III), a monomeric octahedral configuration was postulated and this is realized through the coordination of oxygen atoms of the carboxylic, carbonyl and oxime group of two mono-or di-anion ligands. The dianionic form of the ligand is the result of deprotonation of the carboxylic group and mide-alcohol form of the hydroxamic group. For the sulphate-containing iron(III) complex a dimeric coordination is proposed with two monoanions of the organic ligand (the carbonyl oxygens are not coordinated) and the bridging SO4 group. The relative degree of covalency of the metal-carboxylic oxygen bond is 10.6–45.2% and increases in the order: MnII<FeII<CoII<NiII<ZnII<FeIII. The complexes have been characterized by elemental and t.g. analysis and i.r. spectra.
Similar content being viewed by others
References
A. T. Pilipenko, Organichni reaktivi v neorganichnomu analizi, Vishcha shkola, Kiev, 1972, p. 216.
Khimicheckie dobavki k polimeram, Spravochnik, Khimiya, Moskva, 1973, p. 272.
F. Umland, A. Yansen, D. Tirig and G. Vyunsh,Kompleksnye soedineniya v analiticheskoi khimii, Mir, Moskva, 1975, p. 531.
N. M. Koreman,Organicheskie reagenty v neorganicheskom analize, Khimiya, Moskva, 1980, p. 448.
A. G. Bystrov, V. M. Gal'perin and B. P. Titov,Obezvrezhivanie i utilizatsiya otkhodov proizvodstra plastmass, Khímiya, Leningrad, 1982, p. 264.
E. E. Kriss, I. I. Volchenskova and A. S. Grigor'eva,Koordinatsionnye soedineniya metallov v meditsine, Naukova dumka, Kiev, 1986, p. 215.
V. N. Kosta (Ed.)Obshchii praktikum po organicheckoi khimii, Khimiya, Moskva, 1965, p. 678.
G. Shvarchenbakh and G. Flashka,Kompleksonometricheskoe titrovanie, Khimiya, Moskva, 1970, p. 360.
A. V. Klimova,Osnovnye mikrometody analiza organicheskikh soedinenii, Khimiya, Moskva, 1970, p. 75.
Ya. Yu. Kharitonov and M. A. Sarukhanov,Kolebatel'nye spektry gidroksilamina i ego koordinatsionnykh soedinenii, FAN UzSSR, Tashkent, 1971, p. 191.
T. G. Balicheva and O. A. Lobanova,Elektronnye i kolebatel'nye spektry neorganicheckikh i koordinatsionnykh soedinenii, LGU, Leningrad, 1983, p. 117.
S. Yu. Chundak,Avtoref. kand. diss., Kiev, 1976, p. 22.
P. I. Shman'ko,Avtoref. kand. diss., Kiev, 1977, p. 21.
V. M. Buzash,Avtoref. kand. diss., Kiev, 1979, p. 21.
K. Nakamoto,Infrared And Raman Spectra of Inorganic and Coordination Compounds, Wiley-Interscience, New York 1986, p. 248.
A. A. Pasyntskii, T. Ch. Idrisov, V. M. Novotortsev and V. T. Kalinnikov,Koord. Khimiya,1, 1059 (1975).
A. A. Pasyntskii, T. Ch. Idrisov and K. M. Suvorov,Koord. Khimiya,1, 799 (1975).
M. J. Schmelz, J. Nakagawa, S. Mizushima, J. V. Quagliano,J. Am. Chem. Soc.,81, 287 (1959).
A. Serrei,Spravochik po organicheskim reaktsiyam, Goskhimizdat, Moskva, 1962, p. 299.
K. V. Vatsuro and G. L. Mishchenko,Imennye reaktsii v organicheskoi khimii, Khimiya, 1976, p. 528.
E. S. Demeter, G. I. Danilenko and V. M. Buzash,Ukrainskaya resp. konf. po termicheskomu analizu kompleksnykh soedinenii, Uzhgorod, 1987. g. Synopsis p. 56.
A. Vasserman,Reaktsii Dil'sa-Al'dera, Mir, Moskva, 1968, p. 133.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Midyanko, O.V., Galla, V.Y., Buzash, V.M. et al. Synthesis and physico-chemical characterization of coordination compounds of 3d metals with potassium bicyclo[2.2.1]-hept-5-en-endo-2-oyl-hydroxylamine-3-carboxylate. Transition Met Chem 15, 156–159 (1990). https://doi.org/10.1007/BF01023907
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01023907