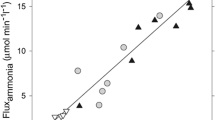
Abstract
Chronic hyperammonemia is known to lead to pathological forms of astrocytes. To test the influence of these changes on the neurotoxicity of ammonia, the glial metabolic poison fluoroacetate (FA) was applied locally, through microdialysis to the hippocampal dentate gyrus. The penetration of ammonia into the brain following the i.p. injection of 7.8 mmol/kg NH4 acetate was evaluated by measuring the ammonia and glutamine content of the microdialysate. Field EPSPs (fEPSPs) evoked by perforant path stimulation were recorded 1.5 mm from the microdialysis probe. When 20 mM FA was perfused, NH4 acetate injection increased the ammonia efflux by 300% and decreased fEPSPs by 40%, but glutamine concentration remained low. With no FA in the microdialysate, NH4 acetate treatment increased the efflux of ammonia by only 60%, did not affect fEPSPs but doubled glutamine efflux. Arterial ammonia content, as measured by microdialysis in the common carotid, increased 4–5 fold following i.p. administration of NH4 acetate, while arterial glutamine was not elevated. Systemically administered FA did not affect either of these changes significantly, but slightly reduced arterial pH. These observations indicate that FA applied by microdialysis acted locally on astrocytes and therefore impaired astrocytic function contributes to the development of hepatic encephalopathy by facilitating the entry of ammonia into the brain. Inhibition of excitatory synaptic transmission by elevated brain ammonia may underlay CNS depression in hepatic encephalopathy.
Similar content being viewed by others
References
Adams, R.D. and Foley, J.M. (1953). The neurological disorder associated with liver disease. In Merit, H.H. and Hare, C.C. (eds.),Metabolic and Toxic Diseases of the Nervous System, Vol. 32, Williams and Wilkins, Baltimore, pp. 198–237.
Aitken, P.G. (1985). Kainic acid and penicillin: differential effects on excitatory and inhibitory interactions in the CA1 region of the hippocampal slice.Brain Res. 325:261–269.
Armitage, P. and Berry, G. (1987).Statistical Methods in Medical Research, 2nd Ed. Blackwell Scientific Publications, Oxford.
Barnes, C.D. and Etherington, L.G. (1964).Drug Dosage in Laboratory Animals, University of California Press, Berkeley and Los Angeles.
Benitez, D., Pscheidt, G.R. and Stone, W.E. (1954). Formation of ammonium ion in the cerebrum in fluoroacetate poisoning.Am. J. Physiol. 176:488–492.
Benveniste, H. (1989). Brain microdialysis.J. Neurochem. 52:1667–1679.
Benveniste, H., Drejer, J., Schousboe, A. and Diemer, N.H. (1984). Elevation of extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis.J. Neurochem. 43:1369–1374.
Benveniste, H., Hansen, A.J. and Ottosen, N.S. (1989). Determination of brain interstitial concentrations by microdialysis.J. Neurochem. 52:1741–1750.
Butterworth, R.F. (1991). Pathophysiology of hepatic encephalopathy: the ammonia hypothesis revisited. In Bengtsson, F., Jeppson, B., Almdal, T. and Vilstrup, H. (eds.).Hepatic Encephalopathy and Metabolic Nitrogen Exchange, CRC Press, Boca Raton, FL. pp. 9–24.
Butterworth, R.F., Girard, G. and Giguère, J.F. (1988). Regional differences in the capacity for ammonia removal by brain following portacaval anastomosis.J. Neurochem. 51:486–490.
Clarke, D.D., Nicklas, W.J. and Berl, S. (1970). Tricarboxylic acid-cycle metabolism in the brain. Effect of fluoroacetate and fluorocitrate on the labeling of glutamate, aspartate, glutamine and γ-aminobutyrate.Biochem. J. 120:345–351.
Cooper, A.J.L., Mora, S.N., Cruz, N.F. and Gelbard, A.S. (1985). Cerebral ammonia metabolism in hyperammonemic rats.J. Neurochem. 44:1716–1723.
Cooper, A.J.L. and Plum, F. (1987). Biochemistry and physiology of brain ammonia.Physiol. Reviews 67:440–519.
Duffy, T.E. and Plum, F. (1982). Hepatic encephalopathy. In Arias I., Popper, H., Schachter, D. and Shafritz, D.A. (eds),The Liver: Biology and Pathobiology, Raven Press, N.Y. pp. 693–715.
Ehrlich, M., Plum, F. and Duffy, T.E. (1980). Blood and brain ammonia concentrations after portacaval anastomosis. Effects of acute ammonia loading.J. Neurochem. 34:1538–1542.
Fan, P., Lavoie, J., Lé, N.L.O., Szerb, J.C. and Butterworth, R.F. (1990). Neurochemical and electrochemical and electrophysiological studies on the inhibitory effect of ammonium ions on synaptic transmission in slices of rat hippocampus: evidence for a postsynaptic action.Neuroscience 37:327–334.
Gibson, G.E., Zimber, A., Krook, L., Richardson, E.P. Jr. and Visek, W.J. (1974). Brain histology and behavior of mice injected with urease.J. Neuropath. Exp. Neurol. 33:201–211.
Gonda, O. and Quastel, J.H. (1966). Transport and metabolism of acetate in rat cortex in vitro. Biochem. J.100:83–94.
Hassel, B., Paulsen, R.E., Johnsen, A. and Fonnum, F. (1992). Selective inhibition of glial cell metabolismin vivo by fluorocitrate.Brain Res. 576:120–124.
Haussinger, D. (1986). Regulation of hepatic ammonia metabolism: the intracellular glutamine cycle.Adv. Enzyme Regul. 25:159–180.
Hawkins, R.A. and Jessy, J. (1991). Hyperammonaemia does not impair brain function in the absence of net glutamine synthesis.Biochem. J. 277:697–703.
Hawkins, R.A., Jessy, J., Mans, A.M. and De Joseph, M.R. (1993). Effect of reducing brain glutamine synthesis on metabolic symptoms of hepatic encephalopathy.J. Neurochem. 60:1000–1006.
Lavoie, J., Giguère, J.F., Pomier Layrargues, G. and Butterworth, R.F. (1987). Activities of neuronal and astrocytic marker enzymes in autopsied brain tissue from patients with hepatic encephalopathy.Met. Brain Dis. 2:283–290.
Loracher, C. and Lux, H.D. (1974). Impaired hyperpolarising inhibition during insulin hypoglycaemia and fluoroacetate poisoning.Brain Res. 69:164–169.
Miyake, T. and Kitamura, T. (1992). Glutamine synthetase immunoreactivity in two types of mouse brain glial cells.Brain Res. 586:53–60.
Morrison, P.F., Bungay, P.M., Hsiao, J.K., Mefford, I.N., Dykstra, K.H. and Dedrick, R.L. (1991). Quantitative microdialysis. In Robinson, T.E. and Justice, J.B., Jr. (eds.),Microdialysis in Neurosciences, Elsevier, Amsterdam, pp. 47–80.
Muir, D., Berl, S. and Clarke, D.D. (1986). Acetate and fluoroacetate as possible metabolic markers for glial metabolismin vivo.Brain Res. 380:336–340.
Norenberg, M.D., Lapham, L.W., Nichols, F.A. and May, A.G. (1974). An experimental model for the study of hepatic encephalopathy.Archs. Neurol. 31:106–109.
Norenberg, M.D. and Martinez-Hernandez, A. (1979). Fine structural localization of glutamine synthetase in astrocytes in rat brain.Brain Res. 161:303–110.
Paulsen, R.E. and Fonnum, F. (1989). Role of glial cells for the basal and Ca2+-dependent K+-evoked release of transmitter amino acids investigated by microdialysis.J. Neurochem. 52:1823–1829.
Paulsen, R.E., Contestabile, A., Villani, L. and Fonnum, F. (1987). Anin vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fiuorocitrate.J. Neurochem. 48:1377–1385.
Paxinos, G. and Watson, C. (1986).The Rat Brain in Stereotaxic Coordinates, 2nd ed., Academic Press, San Diego, CA.
Peters, R.A. (1957). Mechanism of the toxicity of the active constituent ofDichapetalum cymosum and related compounds.Adv. Enzymol. 18:113–159, 1957.
Quastel, J.H. (9174). Metabolic compartmentation in the brain and effect of metabolic inhibitors. In Berl, S., Clarke, D.D. and Schneider, D. (eds.),Metabolic Compartmentation and Neurotransmission, Plenum Press, New York, pp. 337–361.
Raabe, W.A. (1981). Ammonia and disinhibition in cat motor cortex by hypoglycemia.Brain Res. 210:311–322.
Raabe, W. and Onstad, G. (1985). Porta-caval shunting changes neuronal sensitivity to ammonia.J. Neurol. Sci. 71:307–314.
Raichle, M.E. and Larson, K.B. (1981). The significance of the NH3-NH4+ equilibrium on the passage of 13N-ammonia from blood to brain. A new regional residue detection model. Circulation Res.48:913–937.
Saito, T. (1990). Glucose-supported oxidative metabolism and evoked potentials are sensitive to fluoroacetate, an inhibitor of glial carboxylic acid cycle in the olfactory cortex slice.Brain Res. 535:205–213.
SAS Institute Inc.,SAS/STAT User's Guide, Version 6, 4th ed., (1989) Cary, NC.
Szerb, J.C. (1991). Glutamate release and spreading depression in the fascia dentata in response to microdialysis with high K+: role of glia.Brain Res. 542:259–265.
Szerb, J.C. and Butterworth, R.F. (1992). Effect of ammonium ions on synaptic transmission in the mammalian central nervous system.Prog. Neurobiol. 39:135–153.
Szerb, J.C. and Issekutz, B. (1987). Increase in the stimulation-induced overflow of glutamate by fluoroacetate, a selective inhibitor of the glial tricarboxylic cycle.Brain Res. 410:116–120.
Tansey, F.A. Farooq, M. and Cammer, W. (1991). Glutamine synthetase in oligodendrocytes and astrocytes: new biochemical and immunochemical evidence.J. Neurochem. 56:266–272.
Théoret, Y. and Bossu, J.L. (1985). Effects of ammonium salts on synaptic transmission to hippocampal CA1 and CA3 pyramidal cellsin vivo. Neuroscience14:807–821.
Théoret, Y., Davies, M.F., Esplin, B. and Capek, R. (1985). Effects of ammonium chloride on synaptic transmission in the rat hippocampal slice.Neuroscience 14:798–806.
Zieve, L. (1982). Hepatic encephalopathy. In Schiff, L. and Schiff, E.R. (eds.),Diseases of the Liver, Lippincott, Philadelphia, pp. 433–459.
Zieve, L. Lyftogt, C. and Draves, K. (1983). Toxicity of fatty acid and ammonia: interaction with hypoglycemia and Krebs cyle inhibition.J. Lab. Clin. Med. 101:930–939.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Szerb, J.C., Redondo, I.M. Astrocytes and the entry of circulating ammonia into the brain: Effect of fluoroacetate. Metab Brain Dis 8, 217–234 (1993). https://doi.org/10.1007/BF01001063
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01001063