Abstract
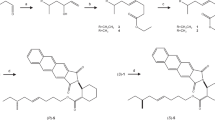
Wittig condensations of aldehydes or ketones with phosphonium salts are frequently used methods for the syntheses of straight-chain or branched alkenes. Suitable choice of reaction conditions may provide the desired geometrical isomer as reaction product. However, we have found that mixtures of geometrical isomers can be conveniently separated on a large scale by the relatively inexpensive method of urea inclusion complex formation. The recovery of both isomers from the separation procedure is almost quantitative. Urea inclusion complexes are formed preferentially withE isomers. Formation of the inclusion complex is affected by the skeletal structure and by the nature of the terminal functional group. By applying this method of separation at a convenient stage, several insect pheromones were prepared without the necessity of a stereoselective step. The technique was used for syntheses of (Z)-11-hexadecenal and (Z)-9-tetradecenal (components of theHeliothis virescens pheromone) and for the synthesis of candidate attractants possessing a trisubstituted double bond.
Similar content being viewed by others
References
Butenandt, A., Hecker, E., Hopp, M., andKoch, W. 1962. Über den Sexuallockstoff des Seidenspinners, IV. Die Synthese des Bombykols und dercis-trans-lsomeren Hexadecadien- (10.12)-ole-(l).J. Liebigs Ann. Chem. 658:39–64.
Fieser, L.F., andFieser, M. 1967. Reagents for Organic Synthesis, Vol. 1, pp. 1262–1265, 4. John Wiley and Sons, New York.
Fujiwara, H., Sato, Y., andNishi, K. 1976. Physico-chemical studies on the sex pheromone of insects. II. Inclusion complexation of sex pheromone ofSpodoptera litura F. with urea.J. Takeda Res. Lab. 35:217–226.
Mitchell, E.R., Jacobson, M., andBaumhover, A.H. 1975.Heliothis spp.: Disruption of pheromonal communication with (Z)-9-tetradecen-1-ol formate.Environ. Entomol. 4:577–579.
Radell, J., andConnolly, J.W. 1960. Urea complexes of partially fluorinated esters.J. Org. Chem. 25:1202–1206.
Ratcliffe, R., andRodehorst, R. 1970. Improved procedure of oxidations with the chromium trioxide pyridine complex.J. Org. Chem. 35:4000–4002.
Roelofs, W.L., Hill, A.S., Cardé, R.T., andBaker, T.C. 1974. Two sex pheromone components of the tobacco budworm moth,Heliothis virescens. Life Sci. 14:1555–1562.
Sonnet, P.E. 1969. Synthesis of theN-isobutylamide of alltrans-2,6,8,10-dodecatetraenoic acid.J. Org. Chem. 34:1147–1149.
Tumlinson, J.H., Hendricks, D.E., Mitchell, E.R., Doolittle, R.E., andBrennan, M.M. 1975. Isolation, identification, and synthesis of the sex pheromone of the tobacco budworm.J. Chem. Ecol. 1:203–214.
Underhill, E.W., Steck, W.F., andChisholm, M.D. 1976. Sex pheromone of the clover cutworm moth.Scotogramma trifolii: Isolation, identification and field studies.Environ. Entomol. 5:307–310.
Author information
Authors and Affiliations
Additional information
This work was conducted under a Cooperative Agreement between the University of Maryland and the Agricultural Research Service.
Mention of a commercial product in this paper does not constitute an endorsement of this product by the USDA.
Rights and permissions
About this article
Cite this article
Leadbetter, G., Plimmer, J.R. An improved preparation of some insect sex attractants: Synthesis and separation of geometrical isomers by formation of urea complexes. J Chem Ecol 5, 101–108 (1979). https://doi.org/10.1007/BF00987691
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00987691