Abstract
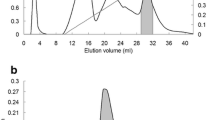
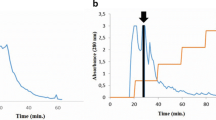
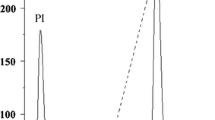
The distribution of lectin in various tissues ofPhaseolus vulgaris L. (cv. red) has been investigated using a sensitive solid-phase enzyme immunoassay. Roots, leaves and stems from 3- to 4-week-old plants were screened for their lectin content; low levels could be detected in all organs, with a relative distribution of 37% in roots, 20% in leaves and 43% in stems. The lectin from stemsleaves and roots was then isolated from 5- to 6-week-old plants using extraction, salt fractionation and affinity chromatography on immobilized porcine thyroglobulin. A comparative study of the seed lectin and the lectin isolated from 5- to 6-week-old plants was made using hemagglutination, inhibition of hemagglutination, immunodiffusion, polyacrylamide and agarose electrophoresis. The results showed that lectin isolated from the different tissues was immunologically identical and exhibited the same subunit structure and similar isolectin composition as the seed lectin.
Similar content being viewed by others
Abbreviations
- EDTA:
-
ethylenediaminetetraacetic acid
- PHA:
-
phytohemagglutinin
- SDS:
-
sodium dodecyl sulfate
References
Borrebaeck, C.A.K., Mattiasson, B. (1979) Recent developments in heterogeneous enzyme immunoassay. J. Solid Phase Biochem.4, 57–67
Borrebaeck, C.A.K., Mattiasson, B. (1983) distribution of a lectin in tissues ofPhaseolus vulgaris. Physiol. Plant.58, 29–32
Bowles, D., Lis, H., Sharon, N. (1979) Distribution of lectins in membranes of soybean and peanut plants. I. General distribution in root, shoot and leaf tissue at different stages of growth. Planta145, 193–198
Etzler, M.E., Borrebaeck, C.A.K. (1980) Carbohydrate binding activity of a lectin-like glycoprotein from stems and leaves ofDolichos biflorus. Biochem. Biophys. Res. Commun.96, 92–97
Felsted, R.L., Leavitt, R.D., Bachur, N.R. (1975) Purification of the phytohemagglutinin family of proteins from the red kidney beans (Phaseolus vulgaris) by affinity chromatography. Biochim. Biophys. Acta405, 72–81
Felsted, R.L., Leavitt, R.D., Chen, C., Bachur, N.R., Dale, R.M.K. (1981) Comparison ofPhaseolus vulgaris cultivars on the basis of isolectin differences. Biochim. Biophys. Acta668, 132–140
Gade, W., Jack, M.A., Dahl, J.B., Schmidt, E.L., Wold, F. (1981) The isolation and characterization of a root lectin from soybean (Glycine max (L.) cultivar Chippewa). J. Biol. Chem.256, 12905–12910
Gatehouse, J.A., Boulter, D. (1980) Isolation and properties of a lectin from the roots ofPisum sativum (garden pea). Physiol. Plant.49, 437–442
Goldstein, I.J., Hughes, R.C., Monsigny, M., Osawa, T., Sharon, N. (1980) What should be called a lectin? Nature (London)285, 66
Harboe, N., Ingild, A. (1973) Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand. J. Immunol. [Suppl. 1]2, 161–164
Horejsi, V., Haskovec, C., Kocourek, J. (1978a) Studies on lectin. XXXVIII. Isolation and characterization of the lectin from black locust bark (Robinia pseudacacia L.). Biochim. Biophys. Acta932, 98–104
Horejsi, V., Chaloupecka, O., Kocourek, J. (1978b) Studies on lectins. XLIII. Isolation and characterization of the lectin from restharrow roots (Ononis hircina jacq). Biochim. Biophys. Acta539, 287–293
Horejsi, V., Kocourek, J. (1978) Studies on lectins. XXXVI. Properties of some lectins prepared by affinity chromatography on O-glycosyl polyacrylamide gels. Biochim. Biophys. Acta538, 299–315
Howard, I.K., Sage, H.J., Horton, C.B. (1972) Studies on the appearance and location of hemagglutinins from a common lentil during the life cycle of the plant. Arch. Biochem. Biophys.149, 323–326
Johansson, B.G. (1972) Agarose gel electrophoresis. Scand. J. Clin. Lab. Invest. [Suppl. 124]29, 7–19
Kohn, J., Wilchek M. (1978) A colorimetric method for monitoring activation of Sepharose by cyanogen bromide. Biochem. Biophys. Res. Commun.84, 7–14
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London)227, 680–685
Leavitt, R.D., Felsted, R.L., Bachur, N.R. (1977) Biological and biochemical properties ofPhaseolus vulgaris isolectins. J. Biol. Chem.252, 2961–2966
Lis, H., Sharon, N. (1981) Lectins in higher plants. In: The biochemistry of plants, vol. 6: Proteins and nucleic acids pp. 371–447, Marcus, A., ed. Academic Press, New York
Lowry, D.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. (1951) Protein measurements with the Folin phenol reagent. J. Biol. Chem.193, 265–275
Mialonier, G., Privat, J.P., Monsigny, M., Kahlem, G., Durand, R. (1973) Isoelement, propriétés, physico-chimiques et localisation in vivo d'une phytohémagglutine (lectine) dePhaseolus vulgaris L. (var. rouge). Physiol. Veg.11, 519–537
Neville, D.M., Jr. (1971) Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J. Biol Chem.246, 6328–6333
Ouchterlony, Ö. (1948) In vitro method for testing the toxinproducing capability of diphtheria bacteria. Acta Pathol. Microbiol. Scand.25, 186–191
Pueppke, S.G., Bauer, W.D., Keegstra, K., Ferguson, A.L. (1978) Role of lectins in plant-microorganism interactions. II. Distribution of soybean lectin in tissues ofGlycine max (L.) Merr. Plant Physiol.61, 779–784
Read, S.M., Northcote, D.H. (1981) Minimization of variation in the response to different proteins of the Coomassie Blue G dye-binding assay for protein. Anal. Biochem.116, 53–64
Rougé, P. (1974a) Etude de la phytohémagglutinine des grains de Lentille au course, de la germination et des premiers stôde du dévéloppement de la plante. Evolution dans les cotylédons. C.R. Acad. Sci. Paris Ser. D278, 449–452
Rougé, P. (1974b) Devenir des phytohémagglutinine provenant des diverses parties de la graine dans les jeunes germinations du pois. C.R. Acad. Sci. Paris Ser. D280, 2105–2108
Su, L.-C., Pueppke, S.G., Friedman, H.P. (1980) Lectins and the soybean-Rhizobium symbiosis. I. Immunological investigations of soybean lines, the seeds of which bave been reported to lack the 120000 daltons soybean lectin. Biochim. Biophys. Acta629, 292–304
Suzuki, I., Saito, H., Inoue, S., Migita, S., Takahashi, T. (1979) Purification and characterization of two lectins fromAloe arborescens Mill. J. Biochem.85, 163–171
Talbot, C.F., Etzler, M.E. (1978a) Development and distribution ofDolichos biflorus lectin as measured by radio immunoassay. Plant Physiol.61, 847–850
Talbot, C.F., Etzler, M.E. (1978b) Isolation and characterization of a protein from stems and leaves ofDolichos biflorus that cross reacts with antibodies to the seed lectin. Biochemistry17, 1471–1479
Toms, G.C., Western A. (1971) Chemotaxonomy of the Leguminosae. Academic Press, New York
Weber, K., Osborn, M. (1969) The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem.224, 4406–4412
Wilson, B., Nakane, P.K. (1978) Recent developments in the periodate method of conjugating horseradish peroxidase (HRPO) to antibodies. In: Immunofluorescence and related staining techniques, pp. 215–224, Knapp, W., Holubar, K., Wick, G., eds. Elsevier/North-Holland Biomedical Press, Amsterdam
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Borrebaeck, C.A.K. Detection and characterization of a lectin from non-seed tissue ofPhaseolus vulgaris . Planta 161, 223–228 (1984). https://doi.org/10.1007/BF00982916
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00982916