Abstract
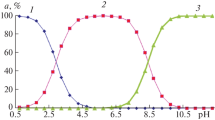
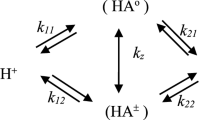
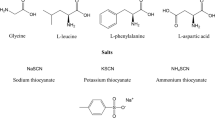
The stoichiometric acid-base equilibrium constants for α-alanine in tetraethylamonium iodide-potassium nitrate solutions were determined at a constant ionic strength of 1.4 m at 25°C. The results obtained are discussed on the basis of the Friedman and the Pitzer model for electrolyte mixtures.
Similar content being viewed by others
References
K. S. Pitzer,Ion Interaction Approach: Theory and Data Correlation in Activity Coefficients in Electrolyte Solution, K.S. Pitzer, ed., (CRC, Boca Raton, Florida, 1991).
K. S. Pitzer,J. Phys. Chem. 77, 268 (1973).
G. Scatchard,J. Am. Chem. Soc. 83, 2636 (1961).
S. Fiol, I. Brandariz, R. Herrero, T. Vilariño, and M. Sastre de Vicente,Ber. Bunsenges. Phys. Chem. 98, 164 (1994).
S. Fiol, F. Arce, X. L. Armesto, F. Penedo, and M. Sastre de Vicente,Fresemius J. Anal. Chem. 343, 469 (1992).
R. Herrero, X. L. Armesto, F. Arce, and M. Sastre de Vicente,J. Solution Chem. 21, 1185 (1992).
R. Herrero, I. Brandariz, and M. Sastre de Vicente,Ber. Bunsenges. Phys. Chem. 97, 59 (1993).
R. Herrero, I. Brandariz, S. Fiol, and M. Sastre de Vicente,Collect. Czech. Chem. Commun. 58, 1269 (1993).
I. Brandariz, F. Arce, X. L. Armesto, F. Penedo, and M. Sastre de Vicente,Monat. Chem. 124, 249 (1993).
I. Brandariz, R. Herrero, and M. Sastre de Vicente,J. Chim. Phys. 90, 63 (1993).
A. Albert and E. P. Serjeant,The Determination of Ionization Constans, 3rd edn. (Chapman and Hall, New York, 1984).
M. Izaguirre and F. J. Millero,J. Solution Chem. 16, 827 (1987).
P. G. Daniele, A. De Robertis, C. De Stefano, S. Sammartano, and C. Rigano,J. Chem. Soc. Dalton Trans. 2353, (1985).
T. H. Lilley,Physical Properties of Amino Acid Solution inChemistry and Biochemistry of the Amino Acids, G. C. Barret, ed., (Chapman and Hall, London, 1985).
H. L. Friedman,Ionic Solution Theory (Interscience-Willey, New York, 1962).
Xu Hua and H. L. Friedman,J. Solution Chem. 19, 1155 (1990).
T. F. Young,Rec. Chem. Proc. 12, 81 (1951).
T. F. Young and M. B. Smith,J. Phys. Chem. 58, 716 (1954).
F. A. Long and W. F. McDevit,Chem. Rev. 51, 119 (1952).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brandariz, I., Fiol, S. & Sastre de Vicente, M. Protonation constants of α-alanine in electrolyte mixtures at constant ionic strength. J Solution Chem 24, 1049–1058 (1995). https://doi.org/10.1007/BF00973521
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00973521