Abstract
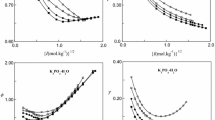
The ternary system LiCl−MgCl2−H2O has been investigated at eight different solution compositions and at 155 5°C. Isopiestic measurements have been made for total equilibrium molalities ranging up to 16.6 mol-kg−1, using MgCl2(aq) as the reference electrolyte. A description of the osmotic coefficients by the ion interaction model of Pitzer gave an overall standard error of 0.006 for the whole data set. The binary Pitzer parameters for MgCl2(aq) were taken from Valyashko and co-workers, whereas the parameters for LiCl(aq) have been determined by the authors in a previous work. It was necessary to use both mixing parameters θMgLi and ψMgLiCl for the fit. The regression gave values of 0.075626 for θMgLi and −7.1392×10−3 for θMgLiCl. The modified B.E.T. equation according to Stokes and Robinson was applied succesfully for water activities lower than 0.4. Using only a linear relationship between the LiCl salt mole fraction and the B.E.T. parameters r and ΔE it was possible to describe the water activities to within 0.36%.
Similar content being viewed by others
References
V. Brendler and W. Voigt,J. Solution Chem.,23, 1061 (1994).
J. A. Rard and R. F. Platford,Activity Coefficients in Electrolyte Solutions, 2nd edn., K. S. Pitzer, (CRC Press, Boca Raton, Florida, 1991).
K. Grjotheim, W. Voigt, B. Haugsdal and A. Dittrich,Acta Chem. Scand. A42, 470 (1988).
K. S. Pitzer,J. Phys. Chem. 77, 268 (1973).
K. S. Pitzer and J. J. Kim,J. Am. Chem. Soc. 96, 5701 (1974).
V. M. Valyashko, M. A. Urusova, W. Voigt and H.-H. Emons,Zh. Neorg. Khim. 33, 228 (1988).
R. G. Anstiss and K. S. Pitzer,J. Solution Chem. 20, 849 (1991).
J. Ananthaswamy and G. Atkinson,J. Chem. Eng. Data 30, 120 (1985).
R. H. Stokes and R. A. Robinson,J. Am. Chem. Soc. 70, 1870 (1948).
M. R. Ally and J. Braunstein,Fluid Phase Equilib. 87, 213 (1993).
W. Voigt,Monatsh. Chem. 124, 839 (1993).
H. Braunstein and J. Braunstein,J. Chem. Thermodyn. 3, 419 (1971).
F.-W. Wollny,Dissertation, Bergakademie Freiberg, 1984.
A. Dittrich,Dissertation, Bergakademie Freiberg, 1986.
J. Sangster, M.-C. Abraham and M. Abraham,J. Chem. Thermodyn. 11, 619 (1979).
M. Abraham,Electrochim. Acta 26, 1397 (1981).
Y. Marcus,Chem. Reviews 88, 1475 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brendler, V., Voigt, W. Isopiestic measurements at high temperature: II. The ternary system LiCl-MgCl2-H2O at 155°C. J Solution Chem 24, 917–924 (1995). https://doi.org/10.1007/BF00973445
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00973445