Abstract
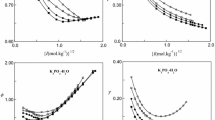
At a temperature of 155.5°C, isopiestic measurements have been made for the ternary system KCl−MgCl2−H2O at 12 different solute mole fractions. Total equilibrium molalities ranged up to 9.71 mol-kg−1. MgCl2(aq) was chosen as the reference electrolyte. A description of the osmotic coefficients by the ion interaction model of Pitzer gave an overall standard error of 0.0177. The binary Pitzer parameters have been taken from Holmes, Mesmer, and Valyashko et al. for KCl(aq) and MgCl2(aq), respectively. It was necessary to use both mixing parametersΘ MgK andΨ MgKCl for the fit, but an additional introduction of an ionic strength dependence for these parameters yielded no further improvement. Due to the solubility limits the modified B.E.T. equation according to Stokes and Robinson could be applied only to a small subset of all data points.
Similar content being viewed by others
References
V. Brendler and W. Voigt,J. Solution Chem. 23, 1061 (1994).
V. Brendler and W. Voigt,J. Solution Chem. 24, 917 (1995).
T. Fanghänel, K. Grjotheim, B. Haugsdal, and W. Voigt,Acta Chem. Scand. 45, 30 (1991).
F.-W. Wollny,Dissertation, Bergakademie Freiberg, 1984.
A. Dittrich,Dissertation, Bergakademie, Freiberg, 1986.
T. Fanghänel and H.-H. Emons,Abh. Sächs. Akad. Wiss., Leipzig 57, 4 (1992).
T. Fanghänel and H.-H. Emons,Z. Anorg. Allg. Chem. 562, 165 (1988).
T. Fanghänel, H.-H. Emons, and K. Köhnke,Z. Anorg. Allg. Chem. 576, 99 (1989).
K. Grjotheim, W. Voigt, B. Haugsdal, and A. Dittrich,Acta Chem. Scand. A42, 470 (1988).
T. Fanghänel, K. Kravchuk, W. Voigt, and H.-H. Emons,Z. Anorg. Allg. Chem. 547, 21 (1987).
V. M. Valyashko, M. A. Urusova, W. Voigt, and H. H. Emons,Zh. Neorg. Khim. 33, 228 (1988).
K. S. Pitzer,J. Phys. Chem. 77, 268 (1973).
K. S. Pitzer and J. J. Kim,J. Am. Chem. Soc. 96, 5701 (1974).
H. F. Holmes and R. E. Mesmer,J. Phys. Chem. 87, 1242 (1983).
K. S. Pitzer,Rev. Mineralogy 17, 97 (1987).
B. Grambow,unpublished database for EQ316 program package, GFS-IFT Braunschweig, 1991.
R. C. Phutela and K. S. Pitzer,J. Solution Chem. 15, 649 (1986).
R. H. Stokes and R. A. Robinson,J. Am. Chem. Soc. 70, 1870 (1948).
M. R. Ally and J. Braunstein,Fluid Phase Equilib. 87, 213 (1993).
W. Voigt,Monatsh. Chem. 124, 839 (1993).
H. Braunstein and J. Braunstein,J. Chem. Thermodyn. 3, 419 (1971).
M.-Ch. Abraham, M. Abraham, and J. Sangster,Can. J. Chem. 56, 348 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brendler, V., Voigt, W. Isopiestic measurements at high temperatures: III. The ternary system KCl−MgCl2−H2O at 155°C. J Solution Chem 25, 83–93 (1996). https://doi.org/10.1007/BF00972761
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00972761