Abstract
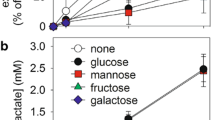
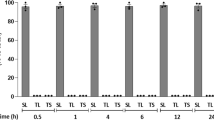
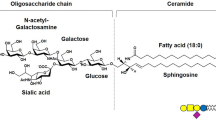
Semisynthetic single-chain GM1 derivatives containing N-acetyl-sphingosine (LIGA4) or N-dichloroacetyl-sphingosine (LIGA20) were recently reported to exert strong protection against glutamate-induced neuronal death in primary cultures of cerebellar granule cells. Elucidation of the molecular mechanism underlying the evoked effect requires knowledge of the metabolic fate of such molecules in the same cultured cells. For this, LIGA4 and LIGA20 were made radioactive on the long chain base moiety and added to cerebellar granule cells in culture in parallel with GM1 ganglioside. The metabolic fate was then investigated. It was found that both these molecules were easily taken up by the cells and promptly metabolized in a fashion qualitatively similar to that of control GM1. The highest amount processed was attributed to the different aggregation properties of LIGAs in solution. Among metabolites, higher accumulation of the single-chain ceramide residues was found after LIGA administration. Interestingly, sphingomyelin was generated, regardless the added compound, suggesting a recycling of the free long chain base.
Similar content being viewed by others
References
Svennerholm, L. 1980. Structure and function of gangliosides. Pp 11–21,in Svennerholm, L., Mandel, P., Dreyfus, H., and Urban, P. F. (eds.), Advances in Exper. Medicine and Biology, Vol. 125, Plenum Press, New York.
IUPAC-IUB Commission on Biochemical Nomenclature, 1977. The nomenclature of lipids. Lipids 12:455–468.
Manev, H., Favaron, M., Vicini, S., Guidotti, A., and Costa, E. 1989. Glutamate-induced neuronal death in primary cultures of cerebellar granule cell: protection by synthetic derivatives of endogenous sphingolipids. J. Pharmacol. Exp. Ther. 252:419–427.
Rothman, S. M., and Olney, J. W. 1987. Excitotoxicity and the NMDA receptor. Trends Neurosci. 10:299–302.
Favaron, M., Manev, H., Alho, H., Bertolino, M., Ferret, B., Guidotti, A., and Costa, E. 1988. Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex. Proc. Natl. Acad. Sci. USA. 85:7351–7355.
Ghidoni, R., Riboni, L., and Tettamanti, G. 1989. Metabolism of exogenous gangliosides in cerebellar granule cells, differentiated in culture. J. Neurochem. 53:1567–1574.
Tettamanti, G., Bonali, F., Marchesini, S., and Zambotti, V. 1973. A new procedure for the extraction, purification and fractionaion of brain gangliosides. Biochim. Biophys. Acta 296:160–170.
Sonnino, S., Kirschner, G., Ghidoni, R., Acquotti, A., and Tettamanti, G. 1985. Preparation of GM1 ganglioside molecular species, having homogeneous fatty acid and long chain base moieties. J. Lipid Res. 26:248–257.
Sarmientos, F., Schwarzmann, G., and Sandhoff, K. 1985. Direct evidence by carbon-13 NMR spectroscopy for the erythro configuration of the sphingoid moiety in Gaucher cerebroside and other natural sphingolipids. Eur. J. Biochem. 146:59–64.
Schwarzmann, G. 1978. A simple and novel method for tritium labeling of gangliosides and other sphingolipids. Biochim. Biophys. Acta. 529:106–114.
Ghidoni, R., Trinchera, M., Venerando, B., Fiorilli, A., Sonnino, S., and Tettamanti, G. 1986. Incorporation and metabolism of exogenous GM1 ganglioside in rat liver. Biochem. J. 237:147–155.
Gallo, V., Ciotti, M. T., Coletti, A., Aloisi, F., and Levi, G. 1982. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc. Natl. Acad. Sci. USA. 79:7919–7923.
Dichter, M. A. 1978. Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 149:279–293.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Sonnino, S., Cantú, L., Corti, M., Acquotti, D., Kirschner, G., and Tettamanti, G. 1990. Aggregational properties of semisynthetic GM1 ganglioside (II3Neu5AcGgOse4Cer) containing an acetyl group as acyl moiety. Chem. Phys. Lipids 56:49–57.
Trinchera, M., Wiesmann, U., Pitto, M., Acquotti, D., and Ghidoni, R. 1988. Different metabolic recycling of the lipid components of exogenous sulphatide in human fibroblasts. Biochem. J. 252:375–379.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pitto, M., Miglio, A., Kirschner, G. et al. Metabolism of semisynthetic single-chain GM1 derivatives in cerebellar granule cells in culture. Neurochem Res 16, 1187–1192 (1991). https://doi.org/10.1007/BF00966694
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00966694