Abstract
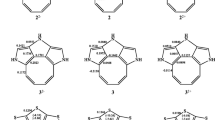
We have determined the spectroscopic characteristics, the dipole moments, and the Kerr constants of the stereoisomers of the secondary-tertiary diols of the cyclohexane and bicyclo [4.1.0]pentane series: 3Β,4α-dihydroxy-3-carane (I), 3Β,4α-dihydroxy-3-methylnorcarane (II), 3α,4Β-dihydroxy-3-methylnorcarane (III), 4α, 5Β-dihydroxy-3-methylcyclohexene (IV), 3Β,4α-dihydroxy-3-carane (V), 3Β,4Β-dihydroxy-3-methylnorcarane (VI), 3α, 4α-dihydroxy-3-carane (VII), and 3α,4α-dihydroxy-4-methyl-3-carane (VIII). It was found that the cis diols are more polar than their trans isomers. It was shown by electrical and electrooptical methods that rotamers with a gauche orientation with respect to the tertiary C-O are stable relative to those containing the diol at the ordinary C-C bond of the ring.
Similar content being viewed by others
Literature cited
P. Buckley, P. A. Giguere, and H. Schneider, Can. J. Chem.,47, No. 6, 901 (1968).
P. -E. Kristiansen, K. -M. Marstokk, and H. Mollendal, Acta Chem. Scand.,A41, No. 7, 403 (1987).
C. Van Alsenoy, L. Van den Enden, and L. Schafer, J. Mol. Struct.,108, No. 112, 121 (1984).
I. P. Povodyreva, Z. G. Isaeva, R. R. Shagidullin, et al., Dokl. Akad. Nauk SSSR,207, No. 4, 908 (1972).
F. W. Nader, W. Heinrich, M. Baar-Schafer, and E. Hangel, Chem. Ber.,118, No. 11, 4314 (1985).
A. N. Vereshchagin, Molecular Polarizability [in Russian], Izd. Nauka, Moscow (1980), pp. 94–100.
A. N. Vereshchagin, Usp. Khim.,52, No. 11, 1879 (1983).
B. A. Arbuzov, V. A. Naumov, Z. G. Isaeva, and R. R. D'yakonova, Dokl. Akad. Nauk SSSR,197, No. 2, 333 (1971).
M. Duin, J. M. A. Baas, and B. Craaf, J. Org. Chem.,51, No. 8, 1298 (1986).
S. Maleknia, B. R. Friedman, N. Abedi, and M. Schwartz, Spectrosc. Lett.,13, 777 (1980).
G. Eglinton, J. Martin, and W. Parker, J. Chem. Soc., No. 2, 1243 (1965).
R. J. Abraham and J. M. Bakke, Acta Chem. Scand.,37B, No. 10, 865 (1983).
Y. Tamura, G. Yamamoto, and M. Oki, Bull. Chem. Soc. Jpn.,60, No. 10, 3789 (1987).
K. B. Wieberg and M. A. Murcko, Mol. Struct. (Theochem.),163, 1 (1988).
é. Kh. Kazakova, L. N. Surkova, and S. V. Andreeva, Izv. Akad. Nauk SSSR, Ser. Khim., No. 7, 1636 (1982).
é. Kh. Kazakova and G. I. Kovylyaeva, Izv. Akad. Nauk SSSR, Ser. Khim., No. 1, 200 (1983).
B. A. Arbuzov, Z. G. Isaeva, R. R. D'yakonova, et al., Izv. Akad. Nauk SSSR, Ser. Khim., No. 7, 1680 (1972).
é. Kh. Kazakova and S. V. Filippova, Izv. Akad. Nauk SSSR, Ser. Khim., No. 12, 2781 (1980).
J. L. Simonsen, J. Chem. Soc., No. 17, 570 (1920).
Z. G. Isaeva, G. Sh. Bikbulatova, O. B. Skripnik, and I. P. Povodyreva, Izv. Akad. Nauk SSSR, No. 5, 1107 (1979).
Author information
Authors and Affiliations
Additional information
Deceased.
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 3, pp. 619–625, March, 1991.
Rights and permissions
About this article
Cite this article
Kazakova, É.K., Andreeva, S.V., Kovylyaeva, G.I. et al. Study of the rotational isomerism about the C-O bond in six-membered secondary-tertiary diols. Russ Chem Bull 40, 538–543 (1991). https://doi.org/10.1007/BF00957991
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00957991