Conclusions
-
1.
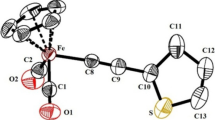
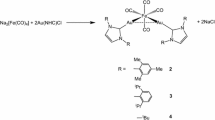
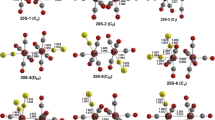
The binuclear complex Fe2-μ-(o-C6H4CH2NC6H5)(CO)6 and the trinuclear complex Fe3-μ-(o-C6H4CH2NC6H5)(CO)8 undergo interconversion by addition and cleavage of iron carbonyl moieties.
-
2.
The arene ligand in Fe3-μ-(o-C6H4CH2NC6H5)(CO)8 is bonded by an exocyclic double bond to one of the iron atoms, and by five π-electrons to the Fe(CO)2 group.
-
3.
Iron carbonyl fragments are readily cleaved from Fe3-μ-(o-C6H4CH2NC6H5)(CO)8 by treatment with sulfur or Fe2(CO)6S2.
Similar content being viewed by others
Literature cited
N. S. Nametkin, V. D. Tyurin, A. I. Nekhaev, et al., J. Organomet. Chem.,243, 323 (1983).
N. S. Nametkin, V. D. Tyurin, V. V. Trusov, et al., J. Organomet. Chem.,276, 199 (1984).
N. A. Vol'kenau, Methods of Heteroorganic Chemistry. π-Complexes of Transition Metals with Dienes, Arenes, and Compounds with M-C o-bonds [in Russian], Moscow (1976), p. 129.
T. V. Nikitina, Methods of Heteroorganic Chemistry. Organometallic Compounds of Iron [in Russian], Nauka, Moscow (1985), pp. 157–158.
A. C. Day and J. T. Powell, J. Chem. Soc. Chem. Commun., 1241 (1968).
E. O. Fischer, W. Berngruber, and C. G. Kreiter, J. Organomet. Chem.,14, 25 (1968).
S. R. Weber and H. H. Brintzinger, J. Organomet. Chem.,127, 45 (1977).
G. G. Aleksandrov, Yu. P. Sobolev, A. I. Nekhaev, et al., Izv. Akad. Nauk SSSR, Ser. Khim., 2534 (1986).
Yu. A. Borisov and I. I. Kritskaya, Izv. Akad. Nauk SSSR, Ser. Khim., 576 (1984).
Yu. V. Chizhov, M. M. Timoshenko, V. I. Kleimenov, et al., Zh. Strukt. Khim.,27, 69 (1986).
K. Ingold, The Theoretical Foundations of Organic Chemistry [Russian translation], Mir, Moscow (1973), p. 181.
A. Solladie-Cavallo, J. Suffert, and A. de Cian, J. Organomet. Chem.,236, 83 (1982).
V. V. Trusov, A. I. Nekhaev, Yu. V. Maksimov, and V. D. Tyurin, Izv. Akad. Nauk SSSR, Ser. Khim., 1903 (1985).
J. A. S. Howell, B. F. G. Johnson, P. L. Josty, and J. Lewis, J. Organomet. Chem.,39, 329 (1972).
N. S. Nametkin, V. D. Tyurin, M. A. Kukina, et al., Izv. Akad. Nauk SSSR, Ser. Khim., 2384 (1977).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 5, pp. 1133–1136, May, 1988.
Rights and permissions
About this article
Cite this article
Nekhaev, A.I., Sobolev, Y.P. & Dorokhina, N.I. Iron carbonyl complexes containing the azomethylene fragment. 6. Interconversion of Fe2-μ-(o-C6H4CH2NC6H5)(CO)6 and Fe3-μ-(o-C6H4CH2NC6H5)(CO)8 . Russ Chem Bull 37, 994–997 (1988). https://doi.org/10.1007/BF00957077
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00957077