Conclusion
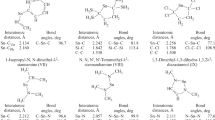
The electron-donating capacities of tin-containing amines depend almost exclusively on the donor-acceptor properties and steric effects of the substituents at the N atom. The effect of dπ-pπ interaction in the Sn-N fragment of the tin-containing amines, unlike the analogous interaction in their silicon and germanium analogs, is hardly apparent. An increase in the number of trialkylstannyl radicals at the N atom leads to a fall in the donor capacity on passing from mono- to bis(trialkylstannyl)amines and to a considerable rise in this capacity in the tris(trialkylstannyl)amines. The donor properties of the dialkylamino-tannanes fall with an increase in the number of amino groups at the Sn atom.
Similar content being viewed by others
Literature cited
E. A. Abel, D. A. Armitage, and D. B. Brady, Trans. Faraday Soc.,62, 3459 (1966).
J. Mack and C. H. Yoder, Inorg. Chem.,8, 278 (1969).
E. W. Randall, C. H. Yoder, and J. J. Zuckerman, Inorg. Chem.,6, 744 (1967).
Z. Pacl, M. Jakoubkova, R. Rericha, and V. Chvalovsky, Collect. Czech. Chem. Commun.,36, 2181 (1971).
A. N. Egorochkin and S. E. Skobeleva, Usp. Khim.,48, 2216 (1979).
A. Marchand, M. T. Torel, and M. Rivere-Baudet, J. Organomet. Chem.,156, 341 (1978).
A. V. Iogansen, in: The Hydrogen Bond [in Russian], Nauka, Moscow (1981), p. 112.
A. G. Davies and T. N. Mitchell, J. Organomet. Chem.,6, 568 (1968).
A. V. Belyakov, L. S. Khaikin, L. V. Vilkov, N. V. Girbasova, E. T. Bogoradovskii, and V. S. Zavgorodnii, Zh. Obshch. Khim.,50, 695 (1979).
É. Lukevits, M. G. Voronkov, E. E. Shestakov, and A. E. Pestunovich, Zh. Obshch. Khim.,41, 2218 (1971).
A. N. Egorochkin, Yu. D. Semchikov, N. S. Vyazankin, and S. Ya. Khorshev, Izv. Akad. Nauk SSSR, Ser. Khim., 152 (1970).
M. R. Kula, C. G. Kreiter, J. Lorberth, Chem. Ber.,97, 1294 (1964).
M. Jones and M. F. Lappert, J. Chem. Soc., J. Chem. Soc., 1944 (1965).
M. E. Bishop and J. J. Zuckermann, Inorg. Chem.,16, 1749 (1977).
K. Sisido and S. Kozima, J. Organ. Chem.,29, 907 (1964).
C. H. Bushweller, W. G. Andersen, P. E. Stevenson, D. L. Burkey, and I. W. O'Neil, J. Am. Chem. Soc.,96, 3892 (1974).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 6, pp. 1294–1298.
Rights and permissions
About this article
Cite this article
Skobeleva, S.E., Egorochkin, A.N., Bogoradovshii, E.T. et al. A study of the electron-donating capacity of tin-containing amines by the IR-spectroscopic method. Russ Chem Bull 31, 1153–1157 (1982). https://doi.org/10.1007/BF00955967
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00955967