Abstract
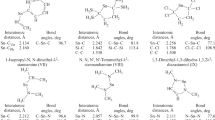
A quantum chemical study of tin–substituent bonds in tricoordinated tin compounds is conducted using the PC GAMESS-Firefly package. The structures of the studied molecules are optimized by the DFT method (B3PW91 functional; aug-cc-pVTZ-pp effective core potential basis set for tin; 6-311++(2d,p) basis set for other atoms). The wave functions and the NBOs of tin–substituent bonds are calculated with HF method using the x2c-TZVPall all-electron relativistic basis set for tin and the 6-311G(2d,2p) basis set for other atoms. Topological characteristics of tin–substituent bonds are calculated with the AIM method. It is shown that these bonds can be referred to as “intermediate type” bonds characterized by small contributions of Sn AOs to the bond MOs, large difference between the charges of tin atoms and the substituent, and low populations of bond MOs. The energies of Sn–R bonds are estimated.











Similar content being viewed by others
REFERENCES
V. Zaitsev, E. A. Kuchuk, A. V. Churakov, G. S. Zaitseva, M. P. Egorov, and S. S. Karlov. Russ. Chem. Bull., Int. Ed., 2017, 66, 622-627. https://doi.org/10.1007/s11172-017-1782-z
T. Chlupatý, Z. Padělková, A. Lyčka, J. Brusc, and A. Růžička. Dalton Trans., 2012, 41, 5010-5019. https://doi.org/10.1039/c2dt12472f
Cambridge Structural Database, release 2021.
M. Veith. Angew. Chem., Int. Ed. Engl., 1987, 26, 1-14. https://doi.org/10.1002/anie.198700013
M. Veith, E. Werle, R. Lisowsky, R. Koppe, and H. Schnockel. Chem. Ber., 1992, 125, 1375-1381. https://doi.org/10.1002/cber.19921250611
M. Veith, M. Jarczyk, and V. Huch. Chem. Ber., 1988, 121, 347-355. https://doi.org/10.1002/cber.19881210222
S. Wang, L. Tao, T. A. Stich, M. M. Olmstead, R. D. Britt, and P. P. Power. Inorg. Chem., 2017, 56, 14596-14604. https://doi.org/10.1021/acs.inorgchem.7b02413
R. Blom and A. Haaland. J. Mol. Struct., 1985, 128, 21-27. https://doi.org/10.1016/0022-2860(85)85036-5
R. J. Gillespie and E. A. Robinson. Chem. Soc. Rev., 2005, 34, 396-407. https://doi.org/10.1039/b405359c
H. Jana, W. Roesky, C. Schulzke, A. Doring, T. Beck, A. Pal, and R. Herbst-Irmer. Inorg. Chem., 2009, 48, 193-197. https://doi.org/10.1021/ic8015639
N. Kuhn, T. Kratz, D. Blaser, and R. Boese. Chem. Ber., 1995, 128, 245-250. https://doi.org/10.1002/cber.19951280307
M. Ozaki, Y. Katsuki, J. Liu, T. Handa, R. Nishikubo, S. Yakumaru, Y. Hashikawa, Y. Murata, T. Saito, Y. Shimakawa, and Y. Kanemitsu. ACS Omega, 2017, 2, 7016-7021. https://doi.org/10.1021/acsomega.7b01292
U. Baumeister, H. Hartung, K. Jurkschat, and A. Tzschach. J. Organomet. Chem., 1986, 304, 107-114. https://doi.org/10.1016/S0022-328X(00)99679-7
N. V. Alekseev and E. A. Chernyshev. J. Struct. Chem., 2008, 49(5), 828-836. https://doi.org/10.1007/s10947-008-0145-x
N. V. Alekseev and E. A. Chernyshev. J. Struct. Chem., 2011, 52(1), 1-8. https://doi.org/10.1134/S002247661101001X
N. V. Alekseev. J. Struct. Chem., 2019, 60(11), 1703-1712. https://doi.org/10.1134/S0022476619110027
N. V. Alekseev. J. Struct. Chem., 2021, 62(3), 185-196. https://doi.org/10.1134/S0022476621020013
A. A. Granovsky. GAMESS Firefly. Version 8.1, 2013. http:// classic.chem.msu.su/gran/firefly/index.html
T. A. Keith. AIMAll. Version 12.11.09. Overland Park, KS, USA: TK Gristmill Software, 2012.
F. W. Biegler-Koning, R. F. Bader, and T. H. Tang. J. Comput. Chem., 1982, 3, 317-321. https://doi.org/10.1002/jcc.540030306
P. L. A. Popelier. MORPHY 98: A Topological Analysis Program. England: UMIST, 1998.
E. D. Glendening, J. K. Badenhoop, A. E. Reed, J. E. Carpenter, J. A. Bohmann, C. M. Morales, and F. Weinhold. NBO 5.0. Natural Bond Orbital Analysis Program. Madison, WI: Theoretical Chemistry Institute, University of Wisconsin, 2001.
C. Boehme and G. Frenking. J. Am. Chem. Soc., 1996, 118, 2039-2046. https://doi.org/10.1021/ja9527075
R. F. W. Bader. Atoms in Molecules: A Quantum Theory. New Nork: Oxford University Press, 1990, 357-386.
I. Alkorta, I. Rozas, and J. Elguero. Struct. Chem., 1998, 9, 243-247. https://doi.org/10.1023/A:1022424228462
K. Zborowskil, I. Alkorta, and J. Elguero. Pol. J. Chem., 2007, 81, 621-629.
W. Nakanishi, S. Hayashi, and K. Narahara. J. Phys. Chem. A, 2009, 113, 10050-10057. https://doi.org/10.1021/jp903622a
E. Espinosa, E. Molins, and C. Lecomte. Chem. Phys. Lett., 1998, 285, 170-173. https://doi.org/10.1016/S0009-2614(98)00036-0
E. Espinosa, L. Alkorta, I. Mata, and E. Molins. J. Phys. Chem. A, 2005, 109, 6532-6534. https://doi.org/10.1021/jp050776s
A. A. Korlyukov, M. Yu. Antipin, N. V. Alekseev, K. V. Pavlov, O. V. Krivolapova, V. G. Lahtin, and E. A. Chernyshev. J. Mol. Struct., 2008, 875, 135-142. https://doi.org/10.1016/j.molstruc.2007.04.019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 4, pp. 410-424.https://doi.org/10.26902/JSC_id89891
Rights and permissions
About this article
Cite this article
Alekseev, N.V. QUANTUM CHEMICAL STUDY OF TIN–SUBSTITUENT BONDS IN TRICOORDINATED TIN COMPOUNDS. J Struct Chem 63, 510–523 (2022). https://doi.org/10.1134/S0022476622040023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622040023