Abstract
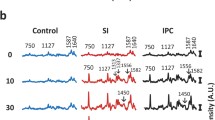
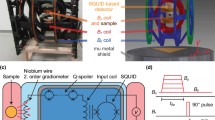
The effects of the cardiac glycoside dihydroouabain (DHO), and the ericaceous toxin grayanotoxin-I (GTX-I) on myocardial cellular sodium (Nai) concentrations were investigated using sodium-23 nuclear magnetic resonance (23Na NMR) spectroscopy at 30°C in isolated perfused guinea-pig hearts. The Nai NMR signals from perfused Langendorff heart preparations were obtained by the modified inversion recovery (IR) method based on the previous observation that the spin-lattice relaxation time (T1) of the Nai (25 or 34 msec at 8.46 Tesla (T)) is much faster than that of extracellular sodium (64 msec at 9.4 T). Nai was estimated from the calibration curve of the frequency area of the23Na NMR FT spectra plotted against the standard Na concentration. The Nai concentration of the heart increased concomitantly with the positive inotropic effects (PIE) of DHO, GTX-I and monensin (MON). The cumulative sequential addition of DHO (5×10−6 M), GTX-I (7×10−8 M) and MON (5×10−6 M), each of which alone induced no appreciable PIE, produced a 22% elevation in Nai concentration relative to that of the control (100%) accompanying a PIE of 44%. The mechanism of this Nai elevation induced by combinational addition of DHO, GTX-I and MON may be mediated as follows: GTX-I increases the net Na-influxvia Na+ channels; DHO inhibits the pumping out of Na+ from the cell; and MON transports external Na+ into the cell, acting as a sodium ionophore. Consequently, these drugs act synergistically to increase the Nai, thereby increasing the intracellular Ca2+ concentrationvia Na+−Ca2+ exchange.
Similar content being viewed by others
References
Lee CO, Kang DH, Sokol JH, Lee KS: Relation between intracellular Na ion activity and tension of sheep cardiac purkinje fibers exposed to dihydro-ouabain. Biophys J 29: 315–330, 1980
Wang DY, Chae SW, Gong QY, Lee CO: Role of aNai in positive forcefrequency staircase in guinea pig papillary muscle. Am J Physiol 255: C798-C807, 1988
Kleber AG: Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res 52: 442–450, 1983
Fortes Mayer KD, Starkey BJ: Simpler flame photometric determination of erythrocyte sodium and potassium: The reference range for apparently healthy adults. Clin Chem 23: 275–278, 1977
Scheufler E, Peters T: Determination of the extracellular space with nonradioactive Co3+ EDTA and simultaneous estimation of Na, K, Ca, and Mg contents in isolated guinea-pig heart preparations by atomic absorption spectrometry. Basic Res Cardiol 82: 341–347, 1987
Macey R, Adorante JS, Orme FW: Erythrocyte membrane potentials determined by hydrogen ion distribution. Biochim Biophis Acta 512: 284–295, 1978
Pike MM, Frazer JC, Dedrick DF, Ingwall JS, Allen PD, Springer CS, Smith TW:23Na and39K nuclear magentic resonance studies of perfused rat hearts. Biophys J 48: 159–173, 1985
Burstein D, Fossel ET: Nuclear magnetic resonance studies of intracellular ions in perfused frog heart. Am J Physiol 252: H1138-H1146, 1987
Fossel ET, Hoefeler H: Observation of intracellular potassium and sodium in the heart by NMR: A major fraction of potassium is ‘invisible’. Magn Reson Med 3: 534–540, 1986
Wittenberg BA, Gupta RJ: NMR studies of intracellular sodium ions in mammalian cardiac myocytes. J Biol Chem 260: 2031–2034, 1985
van Echteld CJ, Kirkels JH, Eijgelshoven, van der Meer P, Ruigrok TJC: Intracellular sodium during ischemia and calcium-free perfusion: A23Na NMR study. J Mol Cell Cardiol 23: 297–307, 1991
Jelicks LA, Gupta RJ: Multinuclear NMR studies of the Langendorff perfused rat heart. J Biol Chem 264: 15230–15235, 1989
Murakami M, Suzuki E, Miyamoto S, Seo Y, Watari H: Direct measurement of K movement by39K NMR in perfused rat mandibular salivary gland stimulated with acetylcholine. Pflügers Arch 414: 385–392, 1989
Seo Y, Murakami M, Watari H: Measurment of intracellular Na in the rat salivary gland: a23Na-NMR study using double quantum filtering. Biochim Biophys Acta 1034: 142–147, 1990
Kuki S, Suzuki E, Watari H, Takami H, Matsuda H, Kawashima Y: Potassium-39 nuclear magnetic resonance observation of intracellular potassium without chemical shift reagents during metabolic inhibition in the isolated perfused rat heart. Circ Res 67: 401–405, 1990
Hotta Y, Takeya K, Kobayashi S, Harada N, Sakakibara J, Shirai N: Relationship between structure, positive inotropic potency and lethal dose of grayanotoxins in guinea pig. Arch Toxicol 44: 259–267, 1980
Shirai N, Sakakibara J, Kaiya T, Kobayashi S, Hotta Y, Takeya K: Quantitative structure-inotropy relationship applied to substituted grayanotoxins. J Med Chem 26: 851–855, 1983
Nakamura A, Nagai S, Ueda T, Sakakibara J, Hotta Y, Takeya K: Studies on the chemical modification of monensin. II. Measurement of sodium ion permeability of monensylamino acids using sodium-23 nuclear magnetic resonance spectroscopy. Chem Pharm Bull 37: 2330–2333, 1989
Pettegrew JW, Woessner dE, Minshew NJ, Glonek T: Sodium-23 NMR analysis of human whole blood, erythrocytes, and plasma. Chemical shift, spin relaxation, and intracellular sodium concentration studies. J Magn Resn 57: 185–196, 1984
Patt SL, Sykes BD: Water eliminated fourier transform NMR spectroscopy. J Chem Phys 56: 3182–3184, 1972
Foy BD, Burstein D: Interstitial sodium nuclear magnetic resonance relaxation times in perfused hearts. Biophys J 58: 127–134, 1990
Burstein D, Fossel ET: Intracellular sodium and lithium NMR relaxation times in the perfused frog heart. Magn Reson Med 4: 261–273, 1987
Springer CS: Measurement of metal cation compartmentalization in tissue by high-resolution metal cation NMR. Ann Rev Biophys Chem 16: 375–399, 1987
Burstein D, Litt HI, Fossel ET: NMR characteristics of ‘Visble’ intracellular myocardial potassium in perfused rat hearts. Magn Reson Med 9: 66–78, 1989
Korth M, Kühlkamp V: Musclarinic receptor-mediated increase of intracellular Na+-ion activity and force of contraction. Pflügers Arch 403: 266–272, 1985
Takeya K, Hotta Y, Yajima M, Sakakibara J: Frequecy-dependent nature of cardiac effect of asebotoxin III. 8th International Congress of Pharmacology, 1981, Abstracts, No. 904. Tokyo
Ku DD, Akera T, Frank M, Brody TM: The effect of grayanotoxin I and α-dihydrograyanotoxin II on guinea-pig myocardium. J Pharmacol Exp Ther 200: 363–372, 1977
Narahashi T, Seyama I: Mechanism of nerve membrane depolarization caused by grayanotoxin I. J Physiol (Lond.) 242: 471–487, 1974
Hotta Y, Ando H, Shirai N, Sakakibara J, Takeya K: Relationship between the inotropy speeds in guinea-pig myocardium and lipophilic character of cardenolides and ericaceous toxins. Japan J Pharmacol 36, 205–215, 1984
Baker PF, Blaustein MP, Hodgkin AL, Steinhart RA: The influence of calcium on sodium efflux in squid axons. J Physiol 200: 431–458, 1969
Glitsch HG, Reuter H, Scholz H: The effect of the internal sodium concentration on calcium fluxes in isolated guinea pig auricles. J Physiol 209: 25–43, 1970
McDonough PM, Goldstein D, Heller Brown J: Elevation of cytoplasmic calcium concentration stimulates hydrolysis of phosphatidylinositol biphosphate in chick heart cells: effect of sodium channel activators. Mol Pharmacol 33: 310–315, 1988
Hugtenburg JG, Mathy M-J, Beckeringh JJ, van Zwieten PA: The differential effect of calcium antagonists on the positive inotropic effects induced by calcium and monensin in cardiac preparations of rats and guinea-pigs. Naunyn-Schmiedeberg's Arch Pharmacol 340: 558–566, 1989
Takeya K, Yajima M, Kobayashi S, Hotta Y, Sakakibara J: Synergistic relation between cardiac glycosides and grayanotoxin I on the positive inotropic effect. Japan J Pharmacol 31 (Suppl. I): 115p, 1981/Yajima M, Hotta Y, Takeya K, Sakakibara J: Altenatively potentiative interrelations on their positive inotropic effects between cardiac glycoside and asebotoxin III: Ibid. Japan J Pharmacol, 36 (Suppl. I): 277P, 1984
Temma K, Akera T: Enhancement of cardiac actions of ouabain and its binding to Na+, K+-adenosine triphosphate by increased sodium influx in isolated guinea-pig heart. J Pharmacol Exp Ther 223: 490–496, 1982
Schwinger RHG, Boehm M, Rosee KL, Schmidt U, Schultz C, Erdmann E: Na+-channel activators increase cardiac glycoside sensitivity in failing human myocardium. J Cardiovasc Pharmacol 19: 554–561, 1992
Reiter M: The positive inotropic action of cardioactive steroids on cardiac ventricular muscle. In: K. Greeff (ed). Handbook of Experimental Pharmacology, 56: Cardiac Glycosides, part I, pp 187–219, Springer-Verlag, 1981
Fricke U, Klaus W: Evidence for two different Na+-dependent(3H)-ouabain binding sites of a Na+, K+-ATPase of guinea-pig hearts. Br J Pharmacol 61: 423–428, 1977
Hotta Y, Nishiyama M, Ando H, Kato M, Takeya K: Different modes of the effects of dihydroouabain and isoproterenol on the fura-2 calcium transients and the contractile response of the isolated guinea-pig heart. J Aichi Med Univ Assoc 17: 911–915, 1989
Simor T, Kim SW, Chu WJ, Pohost GM, Elgavish GA: A selective inversion recovery method for the improvement of23Na NMR spectral resolution in isolated perfused rat hearts. NMR Biomed 6(3): 201–208, 1993
DeLayere JL, Ingwall JS, Malloy C, Fossel ET: Gated sodium-23 nuclear magnetic resonance images of an isolated perfused working rat heart. Science 212: 935–936, 1981
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hotta, Y., Ando, H., Takeya, K. et al. Direct measurement of increased myocardial cellular23Na NMR signals in perfused guinea-pig heart induced by dihydroouabain and grayanotoxin-I. Mol Cell Biochem 139, 59–70 (1994). https://doi.org/10.1007/BF00944204
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00944204