Abstract
The Gram positive anaerobeAcetobacterium woodii is able to grow autotrophically with a mixture of H2 and CO2 as the energy and carbon source. The question, by which pathway CO2 is assimilated, was studied using long term isotope labeling.
Autotrophically growing cultures produced acetate parallel to cell proliferation, and, when U-[14C]acetate was present as tracer, incorporated radioactivity into all cell fractions. The specific radioactivity and the label positions were determined for those representative cell compounds which biosynthetically originated directly from acetyl CoA (N-acetyl groups), pyruvate (alanine), oxaloacetate (aspartate), α-ketoglutarate (glutamate), and hexosephosphates (glucosamine). Per mol compound the same amount of labeled acetate was incorporated into N-acetyl groups, alanine (C-2, C-3), aspartate (C-2, C-3), and twice the amount into glutamate (C-2, C-3, C-4, C-5) and into glucosamine. Consequently, the unlabeled carbon atoms of the C3−C6 compounds must have been derived from CO2 by carboxylation subsequent to acetyl CoA synthesis. When 0.2 mM 2-[14C]pyruvate was added to autotrophically growing cultures, also a substantial amount of radioactivity was incorporated. Two important differences in comparison to the acetate experiment were observed: The N-acetyl groups were almost unlabeled and glutamate contained the same specific radioactivity as alanine or aspartate.
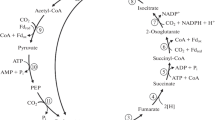
These data showed that acetyl CoA is the central intermediate for biosynthesis and excluded the operation of the Calvin cycle inA. woodii. The results were consistent with the operation of a different autotrophic CO2 fixation pathway in which CO2 is converted into acetyl CoA by total synthesis via methyltetrahydrofolate; acetyl CoA is then further reductively carboxylated to pyruvate.
Similar content being viewed by others
References
Bache R, Pfennig N (1981) Selective isolation ofAcetobacterium woodii on methoxylated aromatic acids and determination of growth yields. Arch Microbiol 130:255–261
Balch WE, Schoberth S, Tanner RS, Wolfe RS (1977)Acetobacterium, a new genus of hydrogen-oxidizing, carbon-dioxide-reducing, anaerobic bacteria. Int J Syst Bacteriol 27:355–361
Bergmeyer HU, Möllering H (1974) Acetat-Bestimmung mit vorgeschalteter Indikatorreaktion. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, Vol 2. Verlag Chemie, Weinheim, pp. 1571–1572
Bernstein IA, Wood HG (1957) Determination of isotopic carbon patterns in carbohydrate by bacterial fermentation. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 4. Academic Press, New York, p 580
Bernt E, Gutmann I (1974) Ethanol-Bestimmung mit Alkohol-Dehydrogenase und DAD. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, Vol 2. Verlag Chemie, Weinheim, pp 1545–1548
Braun K (1979) Untersuchungen zum autotrophen, heterotrophen und mixotrophen Wachstum vonAcetobacterium woodii undClostridium aceticum. Doctoral thesis. University of Göttingen
Braun K, Gottschalk G (1981) Effect of molecular hydrogen and carbon dioxide on chemo-organic growth ofAcetobacterium woodii andClostridium aceticum. Arch Microbiol 128:294–298
Braun M, Mayer F, Gottschalk G (1981)Clostridium aceticum (Wieringa), a microorganism producing acetic acid from molecular hydrogen and carbon dioxide. Arch Microbiol 128:288–293
Daniels L, Fuchs G, Thauer RK, Zeikus JG (1977) Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol 132:118–126
Daniels L, Zeikus JG (1978) One-carbon metabolism in methanogenic bacteria: analysis of short-term fixation products of14CO2 and14CH3OH incorporated into whole cells. J Bacteriol 136:75–84
Decker K (1959) Die aktivierte Essigsäure. Enke Verlag, Stuttgart, pp 81 ff.
Diekert G, Ritter M (1982) Nickel requirement ofAcetobacterium woodii. J Bacteriol 151:1043–1045
Diekert G, Thauer RK (1978) Carbon monoxide oxidation byClostridium thermoaceticum andClostridium formicoaceticum. J Bacteriol 136:597–606
Dorn M, Andreesen JR, Gottschalk G (1978) Fumarate reductase ofClostridium formicoaceticum. Arch Microbiol 119:7–11
Drake HL, Hu SI, Wood HG (1981) Punfication of five components fromClostridium thermoaceticum which catalyze synthesis of acetate from pyruvate and methyltetrahydrofolate. J Biol Chem 256: 11137–11144
Eyzaguirre J, Jansen K, Fuchs G (1982) Phosphoenolpyruvate synthetase inMethanobacterium thermoautotrophicum. Arch Microbiol 132: 67–74
Fischer F, Lieske R, Winzer K (1932) Über die Bildung von Essigsäure bei der biologischen Umsetzung von Kohlenoxyd und Kohlensäure mit Wasserstoff zu Methan. Biochem Z 245:2–12
Fontaine F, Peterson WH, McCoy E, Johnson GJ (1942) A new type of glucose fermentation byClostridium thermoaceticum, n sp J Bacteriol 43:701–715
Fuchs G, Stupperich E (1978) Evidence for an incomplete reductive carboxylic acid cycle inMethanobacterium thermoautotrophicum. Arch Microbiol 118:121–125
Fuchs G, Stupperich E (1980) Acetyl CoA, a central intermediate of autotrophic CO2 fixation inMethanobacterium thermoautotrophicum. Arch Microbiol 127:267–272
Fuchs G, Stupperich E (1982) Autotrophic CO2 fixation pathway inMethanobacterium thermoautotrophicum. Zbl Bakt Hyg I. Abt Orig C 3:277–288
Fuchs G, Stupperich E, Eden G, (1980a) Autotrophic CO2 fixation inChlorobium limicola. Evidence for the operation of a reductive tricarboxylic acid cycle in growing cells. Arch Microbiol 128:64–71
Fuchs G, Stupperich E, Thauer RK (1978) Acetate assimilation and the synthesis of alanine, aspartate and glutamate inMethanobacterium thermoautotrophicum. Arch Microbiol 117:61–66
Fuchs G, Stupperich E, Jaenchen R (1980b) Autotrophic CO2 fixation inChlorobium limicola. Evidence against the operation of the Calvin cycle in growing cells. Arch Microbiol 128:56–63
Gawehn K, Bergmeyer HU (1974)D-(-)Lactat. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, Vol 2. Verlag Chemie, Weinheim, pp 1538–1541
Genthner BRS, Bryant MP (1982) Growth ofEubacterium limosum with carbon monoxide as the energy source. Appl Environmental Microbiol 43:70–74
Gottschalk G (1968) The stereospecificity of the citrate synthase in sulfate-reducing and photosynthetic bacteria. Eur J Biochem 5: 346–351
Grassl M (1974) Alaninbestimmung mit Glutamat-Pyruvat-Transaminase und Lactat-Dehydrogenase. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, Vol 2. Verlag Chemie, Weinheim, pp 1727–1730
Gutmann I, Wahlefeld AW (1974)L-(+)Lactat. Bestimmung mit Lactat-Dehydrogenase und NAD. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse. Verlag Chemie, Weinheim, pp 1510–1514
Herbert D, Phipps PJ, Strange RE (1971) Chemical analysis of microbial cells. In: Norris JR, Ribbons DW (eds) Methods in microbiology, vol 5B. Academic Press, London, New York, pp 209–344
Herrmann WA (1982) Metallorganische Aspekte der Fischer-Tropsch-Synthese. Angew Chem 94:118–131
Hu SI, Drake HL, Wood HG (1982) Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes fromClostridium thermoaceticum. J Bacteriol 149:440–448
Jansen K, Stupperich E, Fuchs G (1982) Carbohydrate synthesis from acetyl CoA in the autotrophMethanobacterium thermoautotrophicum. Arch Microbiol (in press)
Kandler O, König H (1978) Chemical composition of the peptidoglycanfree cell walls of methanogenic bacteria. Arch Microbiol 118:141–152
Kandler O, Schoberth S (1979) Murein structure ofAcetobacterium woodii. Arch Microbiol 120:181–183
Linke HAB (1969) CO2-Fixierung durchClostridium aceticum:14CO2-Kurzzeiteinbau und Pyruvatstoffwechsel. Arch Microbiol 64:203–214
Ljungdahl LG, Wood HA (1969) Total synthesis of acetate from CO2 by heterotrophic bacteria. Ann Rev Microbiol 23:515–538
Lynd L, Kerby R, Zeikus JG (1982) Carbon monoxide metabolism of the methylotrophic acidogenButyribacterium methylotrophicum. J Bacteriol 149:255–263
Oberlies G, Fuchs G, Thauer RK (1980) Acetate thiokinase and the assimilation of acetate inMethanobacterium thermoautotrophicum. Arch Microbiol 128:248–252
Roberts RB, Abelson PH, Cowie DB, Bolton ET, Britton RF (1957) Studies of biosynthesis inEscherichia coli. Carnegie Institute, Washington
Simon H, Floss HG (1967) Anwendung von Isotopen in der organischen Chemie und Biochemie, Vol 2. Springer, Berlin, Heidelberg, New York
Stadtman T (1971) Vitamin B12. Science 171:859–867
Stegemann H (1960) Bestimmung von Aminosäuren mit dithionitreduziertem Ninhydrin. Hoppe-Seyler's Z Physiol Chem 319:102–109
Stupperich E, Fuchs G (1981) Products of CO2 fixation and14C labeling pattern of alanine inMethanobacterium thermoautotrophicum pulselabeled with14CO2. Arch Microbiol 130:294–300
Tanner RS, Stackebrandt E, Fox GE, Woese CR (1981) A phylogenetic analysis ofAcetobacterium woodii, Clostridium barkeri, Clostridium butyricum, Clostridium lituseburense, Eubacterium limosum, andEubacterium tenue. Curr Microbiol 5:35–38
Tanner RS, Wolfe RS, Ljungdahl LG (1978) Tetrahydrofolate enzyme levels inAcetobacterium woodii and their implication in the synthesis of acetate from CO2. J Bacteriol 134:668–670
Taylor GT, Kelly DP, Pirt SJ (1976) Intermediary metabolism in methanogenic bacteria. In: Schlegel HG, Gottschalk G, Pfennig N (eds) Proceedings of the Symposium “Microbial production and utilization of gases (H2, CH4, CO)”. Akademie der Wissenschaften zu Göttingen, E Goltze Verlag, Göttingen, pp 173–180
Thauer RK, Diekert G, Schönheit P (1980) Biological role of nickel. Trends Bioch Sci 5:304–306
Wiegel J, Braun M, Gottschalk G (1981)Clostridium thermoautotrophicum species novum, a thermophile producing acetate from molecular hydrogen and carbon dioxide. Curr Microbiol 5:255–260
Wieringa KT (1940) The formation of acetic acid from carbon dioxide and hydrogen by anaerobic spore-forming bacteria. Antonie van Leeuwenhoek 7. Microbiol Serol 6:251–162
Wood HG, Utter MF (1965) The role of CO2 fixation in metabolism. In: Campbell PN, Greville GD (eds) Essays in biochemistry, vol 1. Academic Press, New York, London, pp 1–27
Zeikus JG, Fuchs G, Kenealy W, Thauer RK (1977) Oxidoreductases involved in cell carbon synthesis ofMethanobacterium thermoautotrophicum. J Bacteriol 132:604–613
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eden, G., Fuchs, G. Total synthesis of acetyl coenzyme a involved in autotrophic CO2 fixation inAcetobacterium woodii . Arch. Microbiol. 133, 66–74 (1982). https://doi.org/10.1007/BF00943772
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00943772