Abstract
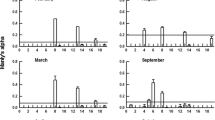
Bottle incubations were conducted in March, July/August and October 1992. to measure the daily rations (R) and objectively characterize the diets of the calanoid copepodsEucalanus elongatus, Undinula vulgaris, Centropages velificatus andTemora stylifera from the west Florida continental shelf. Daily rations,R, were clustered around two, order-of-magnitude different means, 1.3 and 11.2% of body C d−1, representative of quiescent and active feeding modes, respectively. The food concentration at which the transition from quiescent to active mode occurred was influenced by food particle size. In the quiescent mode, diets were dominated by nanoplankton, whereas no food type dominated the diet in the active mode. Selective feeding, defined as a statistically significant difference between the frequency distributions of foods in the diet and environment, occurred in both quiescent and active copepods. However, what appeared to be selective feeding in quiescent copepods could be explained by processes that passively modified the distribution of the diet relative to that of the food supply. Conversely, selective feeding in active copepods apparently resulted from foraging for particles >5 μm in diameter in food environments dominated by nanoplankton (<5 μm).
Similar content being viewed by others
References
Alcaraz M, Paffenhöfer G-A, Strickler JR (1980) Catching the algae: a first account of visual observations of filter-feeding calanoids. In: Kerfoot WC (ed) Evolution and ecology of zooplankton communities. University of New Hampshire, Hanover, pp 241–248
Anderson TR, Hessen DO (1995) Carbon and nitrogen limitation in marine copepods. J Plankton Res 17: 317–331
Bartrum WC (1980) Experimental development of a model for the feeding of neritic copepods on phytoplankton. J Plankton Res 3: 25–51
Cowles TJ (1979) The feeding response of copepod from the Peru upwelling system: food size selection. J mar Res 13: 601–622
Cowles TJ, Strickler JR (1983) Characterization of feeding activity patterns in the planktonic copepodCentropages typicus Kroyer under various food conditions. Limnol Oceanogr 28: 106–116
Dagg M (1977) Some Effets of patchy food environments on copepods. Limnol Oceanogr 22: 99–107
Durbin EG, Durbin AG, Smyada TJ, Verity PG (1983) Food limitation of adultAcartia tonsa in Narragansett Bay, Rhode Island. Limnol Oceanogr 28: 1199–1213
Easterly CO (1916) The feeding habits and food of pelagic copepods and the question of nutrition by organic substances in solution in the water. Univ Calif Publs Zool 16: 171–184
Eppley RW, Sapienza C, Renger EH (1978) Gradients in phytoplankton stocks and nutrients off southern California in 197476. Estuar cstl mar Sci 7: 291–301
Frost BW (1972) Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepodCalanus pac ficus. Limnol Oceanogr 18: 805–815
Frost BW (1977) Feeding behavior ofCalanus pacificus in mixture of food particles. Limnol Oceanogr 22: 472–492
Grice G, Marcus N (1981) Dormant eggs of marine copepods. Oceanogr mar Biol A Rev 19: 125–140
Hakanson JL (1984) Long and short term feeding condition in field caughtCalanus pacificus as determined from lipid content. Limnol Oceanogr 29: 794–804
Hardy AC (1924) The herring in relation to its animate environment. Part I. The food and feeding habits of the herring with special reference to the east coast of England. Fish Invest, Lond (Ser II) 7: 1–53
Hirche HJ (1989) Egg production of the Arctic copepodCalanus glacialis: laboratory experiments. Mar Biol 103: 311–318
Huntley ME, Boyd C (1984) Food limited growth of marine zooplankton. Am Nat 124: 455–478
Jónasdóttir SH, Field D, Pantoja S (1995) Copepod egg production in Long Island Sound, USA, as a function of the chemical composition of seston. Mar Ecol Prog Ser 119: 87–98
Kiørboe T, Mohlenberg F, Nicholajsen H (1982) Ingestion rate and gut clearance in the planktonic copepodCentropages hamatus (Lilleborg) in relation to food concentration and temperature. Ophelia 21: 181–194
Klein Breteler WCM, Schoyt N, Gonzalez SR (1990) On the role of food quality in grazing and development of life stages, and genetic change in body size during cultivation. J exp mat Biol Ecol 135: 177–189
Kleppel GS (1992) Environmental regulation of feeding and egg production byAcartia tonsa off southern California. Mar Ecol Prog Ser 112: 57–65
Kleppel GS (1993) On the diet of calanoid copepods. Mar Ecol Prog Ser 99: 183–195
Kleppel GS, Burkart CA (1995) Egg production and the nutritional environment ofAcartia tonsa: the role of food quality in copepod nutrition. ICES J mar Sci 52: 246–255
Koehl MAR, Strickler JR (1982) Copepod feeding currents: food capture at low Reynolds number. Limnol Oceanogr 26: 1062–1073
Laabir M, Poulet SA, Ianora A (1995) Measuring production of eggs inCalanus helgolandicus. J Plankton Res 17: 1125–1142
Lasker R (1988) Food chains and fisheries: an assessment after 20 years. In: Rothschild BI (ed) Toward a theory on biological-physical interactions in the world ocean. Kluwer, Boston, pp 173–182
Marcus N (1979) On the population biology and nature of diapause ofLabidocera aestiva (Copepoda: Calanoida). Biol Bull mar biol Lab, Woods Hole 157: 297–305
Marshall CM, Orr AP (1955) The biology of the marine copepod,Calanus pacificus (Gunnerus). Oliver and Boyd, Edinburgh
Miller CB, Johnson JK, Heinle DR (1977) Growth rules in the marine copepod genusAcartia. Limnol Oceanogr 22: 326–334
Paffenhöfer G-A (1988) Feeding rates and behavior of zooplankton. Bull mar Sci 43: 430–445
Paffenhöfer G-A, Harris RP (1976) Feeding, growth and reproduction of the marine planktonic copepodPseudocalanus elongatus Boeck. J mar biol Ass UK 56: 327–344
Paffenhöfer G-A, Knowles SC (1980) Omnivorousness in marine planktonic copepods. J Plankton Res 2: 355–365
Pieper RE, Holliday DV, Kleppel GS (1990) Quantitative zooplankton distributions from multifrequency acoustics. J Plankton Res 12: 433–141
Plourde S, Runge JA (1993) Reproduction of the planktonic copepodCalanus finmarchicus in the lower St. Lawrence estuary — relation to the cycle of phytoplankton production and evidence for aCalanus pump. Mar Ecol Prog Ser 102: 217–227
Poulet SA (1974) Seasonal grazing ofPseudocalanus minutus on particles. Mar Biol 25: 109–123
Poulet SA (1978) Comparison between five coexisting species of marine copepods on naturally occurring particulate matter. Limnol Oceanogr 23: 1126–1143
Poulet SA, Ianora A, Miralto A, Meijer L (1994) Do diatoms arrest embryonic development in copepods? Mar Ecol Prog Ser 111: 79–86
Price HJ, Paffenhöfer G-A (1984) Effects of experience in the copepodEucalanus pileatus: a cinematographic study. Mar Biol 84: 35–40
Price HJ, Paffenhöfer G-A (1986) Effects of concentration on the feeding of a marine copepod in algal monocultures and mixtures. J Plankton Res 8: 119–128
Price HJ, Paffenhöfer G-A, Strickler JR (1983) Modes of cell capture in calanoid copepods. Limnol Oceanogr 28: 116–123
Putt M, Stoecker DK (1989) An experimentally determined carbon:volume ratio for marine (Oligotrichous) ciliates from estuarine and coastal waters. Limnol Oceanogr 34: 1097–1130
Raymont JEG (1983) Plankton and productivity in the oceans. Vol. 2. Zooplankton. Pergamon Press, New York
Roman MR (1984) Utilization of detritus by the copepodAcartia tonsa. Limnol Oceanogr 29: 949–959
Ryther JH (1969) Photosynthesis and fish production in the sea. Science 166: 72–76
Schnack SB, Elbrächter M (1983) On the food of calanoid copepods from the northwest Africa upwelling region. In: Richards FA (ed) Coastal upwelling. American Geophysics Union, Washington, DC, pp 433–439
Shannon CE, Weaver W (1949) The mathematical theory of communications. University of Illinois, Urbana
Stoecker DK, Sanders NK (1985) Predation byAcartia tonsa on a dinoflagellate and a tintinnid. J Plankton Res 7: 85–100
Støttrup JG, Jensen J (1990) Influence of algal diet on feeding and egg production of the calanoid copepodAcartia tonsa Dana. J exp mar Biol Ecol 141: 87–105
Strathmann RR (1967) Estimating the organic carbon content of phytoplankton for cell volume or plasma volume. Limnol Oceanogr 12: 411–418
Tester PA, Turner JT (1990) How long does it take copepods to make eggs? J exp mar Biol Ecol 141: 169–182
Turner JT (1984a) The feeding ecology of some zooplankters that are important prey items of larval fish. NOAH tech Rep natn mar Fish Serv 7: 1–28
Turner JT (1984b) Zooplankton feeding ecology: contents of fecal pellets of the copepodsAcartia tonsa andLabidocera aestiva from continental shelf and slope waters near the mouth of the Mississippi River. Pubbl Staz zool Napoli (I. Mar Ecol) 5: 265–282
Turner JT (1984c) Zooplankton feeding ecology: contents of fecal pellets of the copepodsTemora turbinata andT. stylifera from continental shelf and slope waters near the mouth of the Mississippi River. Mar Biol 82: 73–83
Turner JT (1987) Zooplankton feeding ecology: contents of fecal pellets of the copepodCentropages velificatus from waters near the mouth of the Mississippi River. Biol Bull mar biol Lab, Woods Hole 173: 377–386
Urban JL, McKenzie CH, Deibel D (1992) Seasonal differences in the content ofOikopleura vanhoeffeni andCalanus finmarchicus faecal pellets: illustrations of zooplankton food web shifts in coastal Newfoundland waters. Mar Ecol Prog Ser 84: 255–264
Vidal J (1980) Physiology of zooplankton. I. Effects of phytoplankton concentration, temperature and body size on the growth rate ofCalanus pacificus andPseudocalanus sp. Mar Biol 56: 111–134
Wyngaard GA (1988) Geographical variation in dormancy in a copepod: evidence from population crosses. Hydrobiologica 167/169: 367–374
Author information
Authors and Affiliations
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Kleppel, G.S., Burkart, C.A., Carter, K. et al. Diets of calanoid copepods on the West Florida continental shelf: Relationships between food concentration, food composition and feeding activity. Mar. Biol. 127, 209–217 (1996). https://doi.org/10.1007/BF00942105
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00942105