Conclusions
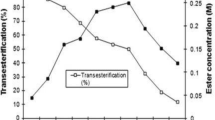
The transesterification of ethyl acetate and butyl butyrate in the presence of sulfuric acid is zero order in ethyl acetate and first order in butyl butyrate. The activation energy of the reaction is 13 kcal /mole. The SNl mechanism was proposed.
Similar content being viewed by others
Literature cited
R. Breslau, Mechanism of Organic Reactions [Russian translation], Mir (1967).
V. V. Korshak and S. V. Vinogradova, Izv. Akad. Nauk SSSR, Otd. Khim. Nauk, 334 (1951).
V. V. Korshak and S. V. Vinogradova, ibid., 179 (1950).
H. Becker, Introduction to Electron Theory of Organic Reactions [Russian translation], Mir (1965).
G. A. Olah, J. Am. Chem. Soc.,89, 5694 (1967).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 7, pp. 1530–1533, July, 1973.
The authors express their gratitude to I. S. Morozova for taking the NMR spectra.
Rights and permissions
About this article
Cite this article
Vedenyapina, N.S., Ivanov, V.V. & Enikolopyan, N.S. Transesterification of ethyl acetate and butyl butyrate in the presence of sulfuric acid. Russ Chem Bull 22, 1483–1485 (1973). https://doi.org/10.1007/BF00930043
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00930043