Abstract
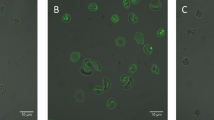
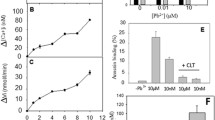
Human and murine blood cells treated with ZnCl2 and bis(sulfosuccinimidyl)suberate (BS3) (a cross linking agent) undergo band 3 clustering and binding of hemoglobin to red blood cell membrane proteins. These clusters induce autologous IgG binding and complement fixation, thus favouring the phagocytosis of ZnCl2/BS3 treated cells by macrophages. The extension of red blood cell opsonization can be easily modulated by changing the ZnCl2 concentration in the 0.1–1.0 mM range thus providing an effective way to affect blood cell recognition by macrophages. In fact, murine erythrocytes treated with increasing ZnCl2 concentrations have proportionally reduced survivals when reinjected into the animal. Furthermore, the organ sequestration of ZnCl2/BS3 treated cells strongly resembles the typical distribution of the senescent cells. Since the ZnCl2/BS3 treatment can also be performed on red blood cells loaded with drugs or other substances, this procedure is an effective drug-targeting system to be used for the delivery of molecules to peritoneal, liver and spleen macrophages.
Similar content being viewed by others
References
Kay MMB: Isolation of the phagocytosis inducing IgG-binding antigen of senescent somatic cells. Nature 289: 491–494, 1981
Kay MMB: Localization of senescent antigen on band 3. Proc Natl Acad Sci USA 81: 5753–5757, 1984
Lutz HU, Stringaro-Wipf G: Senescent red cell-bound IgG is attached to band 3 protein. Biomed Biochim Acta 42: S117-S121, 1984
Khodadad JK, Weinstein RS, Marsh LW, Steck TL: Shape deerminants of McLeod acanthocytes. J Membr Biol 107: 213–218, 1989
Vlassara H: Non-enzymatic tissue glycosylation: mechanism implicated in the complications associated with aging. In: C.E. Finch, T.E. Johnson, (eds.). Molecular biology of aging. Wiley-liss, New York, pp 171–185, 1990
Hui W, Stewart CM, Cherry RJ: Electron microscopic observation of aggregation of membrane proteins in human erythrocyte by mellitin. Biochim Biophys Acta 1023: 335–342, 1990
Lelkes G, Fodor I, Lelkes G, Hollan SR: The mobility of intramembrane particle in non-hemolyzed human erythrocytes factor affecting acridine orange-induced particle aggregation. J Cell Sci 86: 57–67, 1986
Turrini F, Arese P, Yuang J, Low PS: Clustering of integral membrane proteins of human erythrocyte membrane stimulates autologous IgG binding, complement deposition, and phagocytosis. J Biol Chem 266: 23611–23617, 1991
Chiarantini L, Johnson J, Deloach JR: Optimized recirculation survival of mouse carrier erythrocytes. Blood Cells 17: 607–617, 1991
DeLoach JR, Droleskey RE: Survival of murine carrier erythrocytes injected via peritoneum. Comp Biochem Physiol 84A: 447–450, 1986
Magnani M, Rossi L, Brandi G, Schiavano G, Montroni M, Piedimonte G: Targeting antiretroviral nucleoside analogues in phosphorilated form to macrophages:in vitro andin vivo studies. Proc Natl Acad Sci USA 89: 6477–6481, 1992
Magnani M, Rossi L, Bianchi M, Fornaini G, Benatti U, Guida L, Zocchi E, De Flora A: Improved metabolic properties of hexokinase-overloaded human erythrocytes. Biochim Biophys Acta 972: 1–8, 1988
Tonetti M, Astroff B, Satterfield W, De Flora A, Benatti U, DeLoach JR: Construction and characterization of adriamycin-loaded canine red blood cells as a potential slow delivery system. Biotechnol Apll Biochem 12: 621–629, 1990
Naqi A, DeLoach JR, Andrews K, Sattefield W, Keeling M: Determination of parameters for enzymatic therapy using L-asparaginase entrapped in canine erythrocytes. Biotechnol Appl Biochem 10, 365–372, 1988
Mollison PL, Veall N: The use of the isotope51Cr as a label for red cells. Br J Haematol 1: 62–74, 1955
Sluiter W, Oomers LWM, Brand A, Von Furth R: Determination of blood volume in the mouse with chromium-51-labeled erythrocytes. J Immunol Methods 73: 221–226, 1984
Dodge JT, Mitchell C, Hanahan DJ: The preparation and chemical characteristics of hemoglobin free ghosts of human erythrocytes. Arch Biochem Biophys 100: 119–130, 1963
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970
Towbin H, Stachelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354, 1973
Salhany JM, Sloan RJ, Cordes KA:In situ cross-linking of human erythrocyte band 3 by bis(sulfosuccinimidyl)suberate. J Biol Chem 265: 17688–17693, 1990
Lutz HU, Flepp R, Stringaro-Wipf G: Naturally occurring autoantibodies to exoplasmic and cryptic regions of band 3 proteins of human red blood cells. J Immunol 133: 2610–2618, 1984
Salhany JM, Sloan RR: Partial covalent labeling with pyridoxal 5′-phosphate induces bis (sulfosuccinimidyl) suberate crosslinking of band 3 protein tetramers in intact human red blood cells. Biochem Biophys Res Comm 156: 1215–1222, 1988
Benner R, von Oudenaren A, Bjorklund M, Ivars F, Holmberg D: Background immuno globulin production measurement biological significance and regulation. Immunol Today 3: 243–247, 1982
Lutz HU, Wipf G: Naturally occurring autoantibodies to skeletal proteins from human red blood cells. J Immunol 128: 1695–1701, 1982
Low PS, Waugh SM, Zinke K, Drenkhahn D: The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science 227: 531–533, 1985
Valentine WN, Paglia DE: Partial purification of some red blood cell enzymes utilizing hemoglobin precipitation by zinc salts. J Lab Clin Med 101: 617–622, 1983
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chiarantini, L., Rossi, L., Fraternale, A. et al. Modulated red blood cell survival by membrane protein clustering. Mol Cell Biochem 144, 53–59 (1995). https://doi.org/10.1007/BF00926740
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00926740