Conclusions
-
1.
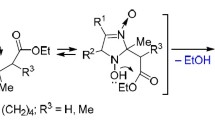
The synthesis of 5-methylphenylamino- and 5-diphenylaminooxazole fromα-N-acylated amino acids and secondary amines of the type C6H5NHR in the presence of dehydrating agents has been carried out.
-
2.
According to the13C NMR and PMR data, the introduction of 5-C6H5NR and (C6H5)2N groups into the oxazole ring results in a significant increase in the screening of the C4 and C2 carbon atoms and the unscreening of the C5 carbon atom.
Similar content being viewed by others
Literature cited
S. M. Birnbaum, L. Levintow, R. B. Kingsley, and J. P. Greenstein, J. Biol. Chem.,194, 455 (1952).
Author information
Authors and Affiliations
Additional information
Translated from IzvestiyaAkademii Nauk SSSR, Seriya Khimicheskaya, No. 4, pp. 894–898, April, 1978.
Rights and permissions
About this article
Cite this article
Kondrat'eva, G.Y., Aitzhanova, M.A., Bogdanov, V.S. et al. Synthesis of 5-R2 N-oxazoles from α-N-acylated amino acids. Russ Chem Bull 27, 773–777 (1978). https://doi.org/10.1007/BF00925305
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00925305