Conclusions
-
1.
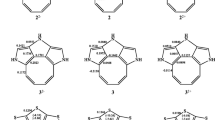
The steric structure of adducts of dichloroketene and cyclohexene, dichloroketene and methyl-cyclohexene, dimethylketene and dihydropyran was investigated by the methods of dipole moments and molar Kerr constants.
-
2.
For all the adducts, the preferential conformation of the bicyclo[4,2,0]octan-7-one system is the anti-boat conformation.
-
3.
The adduct of dimethylketene and dihydropyran has the structure of 8,8-dimethyl-2-oxobicyclo-[4,2,0] octan-7-one. The formation of such a structure is apparently determined by the electron donor influence of the oxygen atom in dihydropyran on the process of cycloaddition.
Similar content being viewed by others
Literature cited
A. P. Krapcho and J. H. Lesser, J. Org. Chem.,31, 2030 (1966).
W. T. Brady and H. R. O'Neal, ibid.,32, 612 (1967).
W. T. Brady, R. Roe, Jr., E. F. Hoff, and F. H. Parry, J. Am. Chem. Soc.,92, 146 (1970).
W. T. Brady and O. H. Woters, J. Org. Chem.,32, 3703 (1967).
J. R. Durig and L. C. Lord, J. Chem. Phys.,45, 61 (1966).
L. H. Sutchiffe and S. M. Walker, J. Phys. Chem.,71, 1555 (1967).
J. M. Conia and J. L. Ripoll, J. Am. Chem. Soc.,84, 4983 (1962).
B. A. Arbuzov, G. G. Butenko, and A. N. Vereshchagin, Dokl. Akad. Nauk SSSR,172, 1323 (1967).
B. A. Naumov and V. M. Bezzubov, Zh. Strukt. Khim.,8, 531 (1967).
R. J. W. Le Fevre, Advanced in Physical Organic Chemistry, V. Gold (editor), Vol. 3, London-New York (1965), p. 1.
M. J. Aroney, D. Izsak, and R. J. W. Le Fevre, J. Chem. Soc., 3997 (1962).
J. B. Hendrickson, J. Am. Chem. Soc., 83, 4537 (1961).
B. A. Arbuzov, S. G. Vul'fson, A. P. Timosheva, and A. N. Vereshchagin, Izv. Akad. Nauk SSSR, Ser. Khim., 1386 (1973).
A. N. Vereshchagin, S. G. Vul'fson, A. A. Solov'ev, and B. A. Arbuzov, ibid., 1639 (1973).
C. G. Le Fevre and R. J. W. Le Fevre, Physical Methods of Organic Chemistry, Vol. 1, Part 3, Chapter 36, New York (1960).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 2, pp. 313–317, February, 1973.
Rights and permissions
About this article
Cite this article
Arbuzov, B.A., Butenko, G.G., Vereshchagin, A.N. et al. Investigation of the steric structure of certain compounds of the bicyclo-[4,2,0]octan-7-one series. Russ Chem Bull 23, 285–288 (1974). https://doi.org/10.1007/BF00924670
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00924670