Abstract
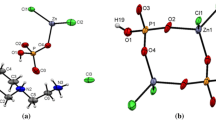
The crystal structure of the adduct tetrachloroethylene carbonate antimony(V)-chloride, C2Cl4O2CO·SbCl5, has been determined by the heavy-atom method from three-dimensional X-ray data. The refinement of the parameters was carried out by least-squares resulting anR-value of 13.1% (space group P21/n — No. 14;a=10.13,b=12.15 andc=12.55 Å, β=108°40′). The unit cell contains 4 discrete molecules C2Cl4O2CO·SbCl5. The antimony atom is coordinated octahedrally by 5 chlorine atoms and the carbonyl oxygen atom of tetrachloroethylene carbonate (Sb−Cl 2.28–2.33 and Sb−O 2.40 Å).
Zusammenfassung
Die Kristallstruktur des Adduktes Tetrachloräthylencarbonat—Antimon(V)-chlorid, C2Cl4O2CO·SbCl5 wurde mit Hilfe der Schweratom-Methode aus dreidimensionalen Röntgendaten bestimmt und nach der Methode der kleinsten Quadrate bis zu einemR-Wert von 13,1% verfeinert (Raumgruppe P21/n — Nr. 14;a=10,13,b=12,15 undc=12,55 Å, β=108°40′). Die Elementarzelle enthält 4 Moleküle C2Cl4O2CO· SbCl5. Das Antimonatom ist oktaedrisch von 5 Chloratomen und dem Carbonylsauerstoffatom des Tetrachloräthylencarbonats umgeben (Sb−Cl 2,28–2,33 und Sb−O 2,40 Å).
Similar content being viewed by others
Literatur
K. Wegleitner, Dissertation Techn. Hochschule Wien, 1969.
V. Gutmann, Angew. Chem.82, 858 (1970); Internat. Ed.9, 843 (1970).
C. I. Brändén undI. Lindqvist, Acta Chem. Scand.17, 353 (1963).
I. Lindqvist, Inorganic Adduct Molecules of Oxo-Compounds. Berlin-Göttingen-Heidelberg: Springer. 1963.
L. Brun undC. I. Brändén, Acta cryst. [Kopenhagen]20, 749 (1966).
J. M. le Carpentier, B. Chevrier undR. Weiss, Bull. Soc. franç. Minér. Crist.91, 544 (1968).
B. Chevrier undR. Weiss, Chem. Comm.4, 145 (1967).
International tables for x-ray crystallography, Vol. 2. Birmingham: The Kynoch Press. 1959.
E. W. Hughes, J. Amer. Chem. Soc.63, 1737 (1941).
M. J. Buerger, X-Ray Crystallography. New York-London-Sydney: Wiley. 1966.
W. C. Hamilton, Acta cryst. [Kopenhagen]18, 502 (1965).
C. J. Brown, Acta cryst. [Kopenhagen]7, 92 (1954).
International tables for x-ray crystallography, Vol. 3. Birmingham: The Kynoch Press. 1962.
Author information
Authors and Affiliations
Additional information
Mit 2 Abbildungen
Rights and permissions
About this article
Cite this article
Kietaibl, H., Völlenkle, H. & Wittmann, A. Die Kristallstruktur von Tetrachloräthylencarbonat—Antimon(V)-chlorid, C2Cl4O2CO·SbCl5 . Monatshefte für Chemie 103, 1360–1376 (1972). https://doi.org/10.1007/BF00904519
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00904519