Abstract
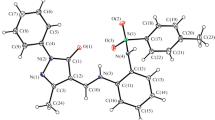
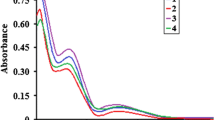
Copper, nickel and iron chelates of pyridine-2.6-di-thiocarbomethylamide were prepared. The discussion about the constitution of the obtained complex compounds is based on magnetic, spectroscopic, thermogravimetric and conductometric studies. 6-Methyl-2-thiocarbomethylamide reacts with copper(II) to give a copper(I) chelate.
Zusammenfassung
Die Komplexbildung von Pyridin-2,6-di-thiocarbomethylamid mit Kupfer-, Nickel- und Eisensalzen wird beschrieben. Die Konstitution der erhaltenen Komplexverbindungen wird an Hand magnetischer, spektroskopischer sowie thermogravimetrischer und konduktometrischer Untersuchungen diskutiert. 6-Methyl-2-thiocarbomethylamid reagient mit Kupfer(II) unter Bildung von Kupfer(I)-Komplexen.
Similar content being viewed by others
Literatur
R. G. Pearson, J. Amer. Chem. Soc.85, 3533 (1963).
A. Spasson, E. Golovinsky undK. J. Markov, C. r. Acad. Sci. de Bulgarie13, 87 (1960);H. Celibonova-Lorer, S. Pančeva-Golovinska undE. Golovinsky, C. r. Acad. Sci. de Bulgarie16, 49 (1963);E. Golovinsky undS. Pančeva-Golovinska, Arzneimittelforsch.18, 1232 (1968).
K. I. Markov, Naturwissensch.47, 450 (1960);K. I. Markov undE. V. Golovinsky, C. r. Acad. Sci. de Bulgarie13, 199 (1960).
G. A. Heath undL. R. Stewart, Austral. J. Chem.22, 83 (1969);K. Knauer, P. Hemmerich undJ. W. D. van Voorst, Angew. Chem.79, 273 (1967);A. Röder undE. Bayer, Angew. Chem.79, 274 (1967).
H. O. Desseyn undM. A. Herman, Spectrochim. Acta23 A, 2457 (1967);H. O. Desseyn, W. A. Jacob undH. A. Herman, Spectrochim. Acta25 A, 1685 (1968).
W. Walter undH. P. Kubersky, Spectrochim. Acta26 A, 1155 (1970).
K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds. Wiley. 1970;H. Siebert, Anwendungen der Schwingungsspektroskopie in der anorg. Chemie. Berlin-Heidelberg-New York: Springer. 1966.
I. Yoe, A. Jones, Ind. Engng. Chem. Anal. Ed.16, 111 (1944).
I. A. McCleverty, Progr. Inorg. Chem.10, 49 (1968), Interscience, New York:E. Hoyer, W. Dietzsch, H. Hennig undW. Schroth, Chem. Ber.102, 603 (1969).
R. N. Sylva undH. A. Goodvin, Austral. J. Chem.21, 1081 (1968).
E. Bayer, Angew. Chem.76, 76 (1964).
R. W. Kluiber, Inorg. Chem.4, 829 (1965).
B. N. Figgis undJ. Lewis, Technique of Inorg. Chemistry, Vol. IV, p. 137, Interscience, New York 1965.
B. Emmert undM. Groll, Chem. Ber.86, 205, 208 (1953).
R. Wegler, E. Kühle undW. Schäfer, Angew. Chem.70, 351 (1958).
Author information
Authors and Affiliations
Additional information
Herrn Prof. Dr.Otto Kratky zum 70. Geburtstag gewidmet.
Mit 3 Abbildungen
Rights and permissions
About this article
Cite this article
Gagliardi, E., Popitsch, A. Über das koordinationschemische Verhalten von Pyridin-2,6-di-thiocarbomethylamid. Monatshefte für Chemie 103, 1337–1348 (1972). https://doi.org/10.1007/BF00904517
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00904517