Abstract
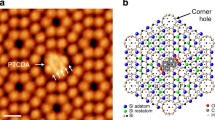
Scanning tunneling microscopy (STM) is used to observe, at the atomic scale, Cr(III) adsorbed to hematite (001) surfaces from aqueous solution. The Cr(III) adsorbates are relatively immobile, but estimated activation energies for surface self-diffusion are lower than those for water or hydroxyl substitution in aqueous Cr(III). Possible causes are effects of STM imaging (artifacts), high ligand-substitution rates for adsorbed species, or participation of substrate Fe (III) ligand exchange. STM imaging of suitable aqueous surface complexes is shown to be feasible, and constitutes a new way to study the relationships between microscopic and macroscopic chemical behavior of adsorbed species in aqueous systems.
Similar content being viewed by others
References
Blum, A.E. and A.C. Lasaga, 1991. The role of surface speciation in the dissolution of albite. Geochim. Cosmochim. Acta 55:2193–2201.
Burton, W.K., N. Cabrera and F.C. Frank, 1951. The growth of crystals and the equilibrium structure of their surfaces. Philos. Trans. Royal Soc. London, Ser. A, 243:299–358.
Casey, W.H. and H.R. Westrich, 1991. Control of dissolution rates of orthosilicate minerals by divalent metal-oxygen bonds. Nature 355:157–159.
Cotton, F.A. and G. Wilkinson, 1988. Advanced Inorganic Chemistry (fifth edition) John Wiley, N.Y., 1455 pp.
Charlet, L. and A. Manceau, 1992. X-ray absorption spectroscopic study of the sorption of Cr(III) at the oxide-water interface. J. Colloid Int. Sci. 148(2):443–458.
Dunphy, J.C., P. Sautet, D.F. Ogletree, O. Dabbousi and M.B. Salmeron, 1993. Scanning tunneling microscopy study of the surface diffusion of sulfur on Re(0001). Phys. Rev. B 47(4):2320–2328.
Dzombak, D.A. and F.M.M. Morel, 1990. Surface Complexation Modeling: Hydrous Ferric Oxide. John Wiley, N.Y., 393 pp.
Eggleston, C.M. and M.F. Hochella Jr., 1990. Scanning tunneling microscopy of sulfide surfaces. Geochim. Cosmochim. Acta 54:1511–1517.
Eggelston, C.M. and M.F. Hochella Jr., 1992. The structure of hematite (001) surfaces by scanning tunneling microscopy: Image interpretation, surface relaxation, and step structure. Am. Mineral. 77:911–922.
Furrer, G. and W. Stumm, 1986. The coordination chemistry of weathering: I. Dissolution kinetics ofδ-Al2O3 and BeO. Geochim. Cosmochim. Acta 50:1847–1860.
Ganz, E., S.K. Theiss, I.S. Hang and J. Golovchenko, 1992. Direct measurement of diffusion by hot tunneling microscopy: Activation energy, anisotropy, and long jumps. Phys. Rev. Lett. 68(10):1567–1570.
Gratz, A.J., S. Manne and P.K. Hansma, 1991. Atomic force microscopy of atomic-scale ledges and etch pits formed during dissolution of quartz. Science 251:1343–1346.
Gratz, A.J., P.E. Hillner and P.K. Hansma, 1993. Step dynamics and spiral growth on calcite. Geochim. Cosmochim. Acta 57:491–495.
Hachiya, K., M. Sasaki, T. Ikeda, N. Mikami and T. Yasunaga, 1984. Static and kinetic studies of adsorption-desorption of metal ions on aγ-Al2O3 surface. 2. Kinetic study by means of pressure jump technique. J. Phys. Chem. 88:27–31.
Hwang, I.-S. and J. Golovchenko, 1992. Observation of metastable structural excitations and concerted atomic motions on a crystal surface. Science, 258:1119–1122.
Kieber, R.J. and G.R. Helz, 1992. Indirect photoreduction of aqueous chromium(VI). Environ. Sci. Technol. 26:307–312.
Marichev, V.A., 1991. A new possibility of application of electron tunneling effects in electrochemical double layer structure investigations. Surf. Sci. 250:220–228.
Merchant, P., R. Collins, R. Kershaw, K. Dwight and A. Wold, 1979. The electrical, optical and photoconducting properties of Fe2−xCrxO3 (0≤ x ≤ 0.47). J. Sol. State Chem. 27:307–315.
Pulfer, K., P.W. Schindler, J.C. Westall and R. Grauer, 1984. Kinetics and mechanism of dissolution of bayerite (γ-Al(OH)3) in HNO3-HF solutions at 298.2 K. J. Coll. Int. Sci. 101:554–564.
Rohrer, G.S., V.E. Henrich, D.A. Bonnell, 1992. A scanning tunneling microscopy and spectroscopy study of the TiO2−x(110) surface. Surf. Sci. 278:146–156.
Rotzinger, F.P., H. Stunzi and W. Marty, 1986. Early stages of the hydrolysis of chromium(III) in aqueous solution. 3. Kinetics of dimerization of the deprotonated aqua ion. Inorg. Chem. 25:489–495.
Schindler, P.W., 1981. Surface complexes at oxide-water interfaces. In: M.A. Anderson and A.J. Rubin (eds.), Adsorption on Inorganics at Solid-Liquid Interfaces, Ann Arbor Science, Ann Arbor, MI, pp. 1–49.
Schindler, P.W. and W. Stumm (1987) The surface chemistry of oxides, hydroxides, and oxide minerals. In: W. Stumm (ed.), Aquatic Surface Chemistry, John Wiley and Sons, N.Y., pp. 83–110.
Somorjai, G.A., 1981. Chemistry in Two Dimensions: Surfaces. Cornell University Press, Ithaca, N.Y., 575 pp.
Sverjensky, D.A., 1992. Linear free energy relations for predicting dissolution rates of solids. Nature 358:310–313.
Sverjensky, D.A., 1993. A physical surface-complexation theory for sorption at the mineral-aqueous solution interface (abstr.). Trans. Am. Geophys. Union 74(16):319.
Wehrli, B., S. Ibric and W. Stumm, 1990. Adsorption kinetics of vanadyl(IV) and chromium(III) to aluminum oxide: Evidence for a two-step mechanism. Coll. and Surf. 51:77–88.
Wieland, E., B. Wehrli and W. Stumm, 1988. The coordination chemistry of weathering: III. A generalization on the dissolution rates of minerals. Geochim. Cosmochim. Acta 52:1969–1981.
Zangwill, A., 1988. Physics at Surfaces. Cambridge University Press, 454 pp.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eggleston, C.M. Direct scanning tunneling microscope (STM) observation of Cr(III) complexes on hematite (001) surfaces. Aquatic Science 55, 240–249 (1993). https://doi.org/10.1007/BF00877269
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00877269