Conclusions
-
1.
The reaction of the π-cyclopentadienyltungsten tricarbonyl anion withβ-chlorovinyl ketones gave σ-RCOCH=CHW(CO)3·C5H5, the structure of which was confirmed by their IR and NMR spectra.
-
2.
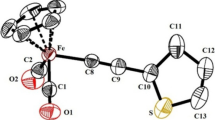
A study was made of the reaction of C6H5COCH=CHW(CO)3C5H5 with iron nonacarbonyl. The following compounds were obtained for the first time: (σ-β-benzoylvinyl-π-cyclopentadienyltungsten tricarbonyl) iron tetracarbonyl (C6H5COCH=CHW(CO)3C5H5)Fe(CO)4; (σ-β-benzoylvinyl-π-cyclopentadienyltung-sten tricarbonyl)iron tricarbonyl (C6H5COCH=CHW(CO)3C5H5)Fe(CO)3, and\(H_2 C = HC - \begin{array}{*{20}c} {//} \hfill & { - - } \hfill & {\backslash \backslash } \hfill \\ \backslash \hfill & { - - - - } \hfill & / \hfill \\ \end{array} - (CH_2 )_n - \begin{array}{*{20}c} {//} \hfill & { - - } \hfill & {\backslash \backslash } \hfill \\ \backslash \hfill & { - - - - } \hfill & / \hfill \\ \end{array} - CH = CH_2 \)
Similar content being viewed by others
Literature cited
A. N. Nesmeyanov, L. V. Rybin, M. I. Rybinskaya, V. S. Kaganovich, Yu. A. Ustynyuk, and I. F. Leshcheva, Zh. Obshch. Khim.,38, 1471 (1968).
R. B. King, Trans. New York Acad. Sci.,28, No.7, 889 (1966).
R. E. Dessy, R. L. Pohl, and R. B. King, J. Am. Chem. Soc.,28, 5121 (1966).
A. N. Nesmeyanov, L. V. Rybin, M. I. Rybinskaya, and Yu. A. Ustynyuk, Zh. Obshch. Khim.,87, 1587 (1967).
R. B. King and M. B. Bisnette, J. Organometal. Chem.,2, 15 (1964).
M. L. H. Green, M. Ishaq, and R. N. Whiteley, J. Chem. Soc. A, 1508 (1967).
A. N. Nesmeyanov, L. V. Rybin, M. I. Rybinskaya, N. T. Gubenko, I. F. Leshcheva, and Yu. A. Ustynyuk, Izv. Akad. Nauk SSSR, Ser. Khim., 1242 (1969).
R. B. King, P. M. Treichel, and F. G. A. Stone, Chem. Ind. (London), 747 (1961).
Author information
Authors and Affiliations
Additional information
See [1] for Communication 1.
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 2, pp. 348–352, February, 1971.
Rights and permissions
About this article
Cite this article
Nesmeyanov, A.N., Rybin, L.V., Rybinskaya, M.I. et al. Synthesis of binuclear complexes of transition metals. Russ Chem Bull 20, 283–286 (1971). https://doi.org/10.1007/BF00869025
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00869025