Conclusions
-
1.
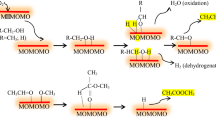
The activation energies of the dehydrogenation and dehydration of isopropanol on ferrite are close, although the dehydrogenation reaction considerably predominates.
-
2.
The low activation energy of the dehydrogenation of tetralin is explained by the fact that cubic structures of nickel ferrite and nickel oxide, promoting the dehydrogenation of tetralin, are formed when the catalyst is calcined. Cracking of tetralin occurs together with the dehydrogenation.
Similar content being viewed by others
Literature cited
W. Selwood, Magnetochemistry, 380, N.J. (1956).
A. A. Balandin, A. A. Tolstopyatova, and Z. Dudzik, Kinetika i Kataliz,2, 273 (1961).
A. A. Balandin, Zh. Obshch. Khimii,16, 793 (1946).
V. N. Kondrat'ev, Uspekhi Khimii,26, 861 (1957).
G. I. Levi and A. A. Balandin, Izv. AN SSSR. Otd. khim. n.,1960, 157.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dudzik, Z., Vol'ski, V. Catalytic properties of ferrites in dehydrogenation and dehydration reactions. Report 1. Transformation of isopropanol and tetralin. Russ Chem Bull 13, 249–252 (1964). https://doi.org/10.1007/BF00853680
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00853680