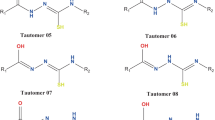
Summary
The 1,6-dioxo-tautomer of hypericin was obtained by basic and BF3 catalyzed tautomerization of the natural and most stable 7,14-dioxo-tautomer. The isolation of this tautomer was aided by its insolubility in methanol. It was identified and characterized by spectroscopic methods, and its detailed structure was derived by means of force field calculations.
Zusammenfassung
Das 1,6-Dioxo-Tautomere des Hypericins wurde durch basen- und BF3-katalysierte Tautomerisierung des natürlichen, stabilen 7,14-Dioxo-Tautomers erhalten. Dessen Isolierung wurde durch die Schwerlöslichkeit in Methanol ermöglicht. Es wurde durch spektroskopische Methoden identifiziert und charakterisiert, und seine detaillierte Struktur wurde aus Kraftfeld-Rechnungen abgeleitet.
Similar content being viewed by others
References
Roth L. (1990) Hypericum-Hypericin, Botanik Inhaltsstoffe Wirkung. Ecomed, Landsberg
Walker E. B., Lee T. Y., Song P. S. (1979) Biochim. Biophys. Acta587: 129; Song P. S. (1981) Biochim. Biophys. Acta639: 1; Kim I., Prusti R. K., Song P. S., Häder D.-P., Häder M. (1984) Biochim. Biophys. Acta799: 298
Meruelo D., Lavie G., Lavie D. (1988) Proc. Natl. Acad. Sci. USA85: 5230; Lavie G., Valentine F., Levin B., Mazur Y., Gallo G., Lavie D., Weiner D., Meruelo D. (1989) Proc. Natl. Acad. Sci. USA86: 5963; Tang J., Colacino J. M., Larsen S. H., Spitzer W. (1990) Antiviral Research13: 313; Lavie D., Revel M., Rotmann D., Van de Velde V. (1988) European Patent Appl. 0256452, A2; Lopez-Bazzocchi I., Hudson J. B., Towers G. H. N. (1991) Photochem. Photobiol.54: 95
Brockmann H., Falkenhausen E. H., Neeff R., Dorlan A., Budde G. (1950) Naturwiss.37: 540; Brockmann H., Kluge F. (1951) Naturwiss.38: 141; Brockmann H., Kluge F., Muxfeldt H. (1957) Chem. Ber.90: 2302; Brockmann H. (1957) Fortschr. Chem. Org. Naturst.14: 141
Falk H., Schoppel G. (1992) Monatsh. Chem.123: 931
Etzlstorfer C., Falk H., Müller N., Schmitzberger W., Wagner U. G. (1993) Monatsh. Chem.124: 751
Falk H., Meyer J. (1992) Hitherto unpublished results
Randic M. (1975) Tetrahedron31: 1477
Falk H., Schmitzberger W. (1992) Monatsh. Chem.123: 731
Falk H., Schlederer T. (1979) Ann. Chem.1979: 1560
Falk H., Meyer J., Oberreiter M. (unpublished)
Sprague J. T., Tai J. C., Yuh Y., Allinger N. L. (1987) J. Comp. Chem.8: 581; Liljefors T., Tai J. C., Yuh Y., Allinger N. L. (1987) J. Comp. Chem.8: 1051
Etzlstorfer C., Falk H., Müller N. (1993) Monatsh. Chem.124: 431
Ball ⇐p; Stick 3.5: Müller N., Falk A. (1992) Cherwell Scientific Publ. Ltd., Oxford, U.K.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Etzlstorfer, C., Falk, H. & Oberreiter, M. On the tautomerism of hypericin: The 1,6-dioxo tautomer. Monatsh Chem 124, 923–929 (1993). https://doi.org/10.1007/BF00816415
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00816415