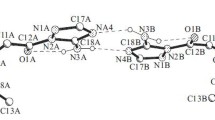
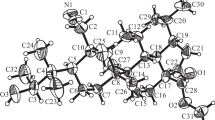
Summary
Optically pure available lactones1 and5 were diastereoselectively oxidised tocis-diols2 and6 by KMnO4 and to epoxides3 and7 by 3-chloroperoxybenzoic acid. Epoxide3 was cleaved totrans-diol4, whereas hydrolysis of7 afforded tricyclic carboxylic acid8. Optic ally puredihydroxylactones2,4, and6 are valuable models for structure determination of the antimicrobial garlic component garlicin.
Zusammenfassung
Die in enatiomerenreiner Form verfügbaren Lactone1 und5 wurden durch diastereoselektive Oxidation mit KMnO4 zu dencis-Diolen2 und6 bzw. mit 3-Chlorperoxybenzoesäure zu den Epoxiden3 und7 umgesetzt. Das Epoxid3 liefert bei der Hydrolyse dastrans-Diol4, während aus7 die tricyclische Carbonsäure8 entsteht. Die optisch reinen Dihydroxylactone2,4 und6 können als Vergleichssubstanzen zur Strukturaufklärung des antimikrobiellen Knoblauchinhaltsstoffes Garlicin dienen.
Similar content being viewed by others
References
Mostler U., Urban E. (1989) Monatsh. Chem.120: 349
Stejskal R., Urban E., Völlenkle H. (1991) Monatsh. Chem.122: 145
Aufreiter U., Fuchs M., Urban E. (1992) Arch. Pharm. (Weinheim)325: [Ph 980]
Jakovac I. J., Goodbrand H. B., Lok K. P., Jones J. B. (1982) J. Am. Chem. Soc.104: 4659
Lok K. P., Jakovac I. J., Jones J. B. (1985) J. Am. Chem. Soc.107: 2521
Gais H. J., Lukas K. L. (1984) Angew. Chem.96: 140
Metz P. (1989) Tetrahedron45: 7311
Storme P., Quaeghebeur L., Vandewalle M. (1984) Bull. Soc. Chim. Belg.93: 999; CA103: 22341
Mohr P., Waespe-Sarcevic N., Tamm C. (1983) Helv. Chim. Acta66: 2501
Takano S., Kurotraki A., Ogasawara K. (1987) Synthesis 1075
Zwergal A. (1952) Pharmazie7: 245
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Edelsbacher, A., Urban, E. & Weidenauer, W. Preparation of enantiomerically pure 5,6-dihydroxy-isobenzofuranones and 5,6-dihydroxy-4,7-methano-isobenzofuranones. Monatsh Chem 123, 741–747 (1992). https://doi.org/10.1007/BF00812323
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00812323