Abstract
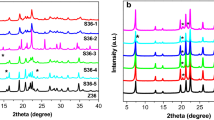
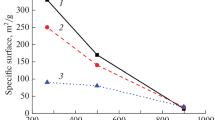
Highly crystalline cobalt aluminophosphates of type 36 have been synthesized and characterized. Investigations on the thermal decomposition of Pr3N-CoAPO-36 and the surface, sorption, acid strength distribution, acidic and catalytic properties of CoAPO-36 were carried out. The XPS analysis indicated that the concentration of cobalt was higher in the bulk of the material than on the surface. The surface of the cobalt aluminophosphate is aluminium rich. The number of strong acid sites is higher on the CoAPO-36 than on CoAPO-5, MAPO-5 and ZAPO-36. The catalytic activities of CoAPO-5, MAPO-5, ZAPO-36, CoAPO-36, MAPO-36 and MAPSO-36 in the 3-methylpentane ando-xylene conversion reactions were compared. The catalytic turnover rate per framework substituted atom in the conversion ofo-xylene for CoAPO-36 is higher than for the MAPO-5, CoAPO-5, ZAPO-36 and MAPSO-36. In the ethylbenzene conversion reaction, the deactivation of the cobalt aluminophosphate and magnesium aluminophosphates of type 36 were studied.
Similar content being viewed by others
References
E.M. Flanigen, B.M. Lok, R.L. Patton and S.T. Wilson, Pure Appl. Chem. 58 (1986) 1351.
E.M. Flanigen, B.M. Lok, R.L. Patton and S.T. Wilson,Proc. 7th Int. Zeolite Conf. (Kodansha/Elsevier, Tokyo/Amsterdam, 1986) p. 103.
E.M. Flanigen, R.L. Patton and S.T. Wilson, Stud. Surf. Sci. Catal. 37 (1988) 13.
S.T. Wilson and E.M. Flanigen, ACS Symp. Ser. 398 (1989) 329.
D.B. Akolekar, J. Catal. 144 (1993) 148.
D.B. Akolekar and S. Kaliaguine, J. Chem. Soc. Faraday Trans. 89 (1993) 4141.
D.B. Akolekar, Zeolites 14 (1994) 53.
J.V. Smith, J.J. Pluth and K.J. Andries,Atlas of Zeolite Structure Types, 3rd rev. Ed., eds. W.M. Meir and D.H. Olson (Butterworth-Heinemann, London, 1992) p. 50.
J.V. Smith, J.J. Pluth and K.J. Andries, Zeolites 13 (1993) 166.
D.B. Akolekar, J. Catal. 143 (1993) 148.
D.B. Akolekar, Zeolites, submitted.
D.B. Akolekar, Appl. Catal. (1994), in press.
V.S. Nayak and V.R. Choudhary, Appl. Catal. 4 (1982) 333.
V.S. Nayak and V.R. Choudhary, J. Catal. 81 (1983) 26.
J.W. Ward, in:Zeolite Chemistry and Catalysis, ACS Monograph No. 171, ed. J.A. Rabo (Am. Chem. Soc., Washington, 1976) p. 118.
V.R. Choudhary and D.B. Akolekar, J. Catal. 119 (1989) 525.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Akolekar, D.B. Investigations on the CoAPO-36 molecular sieve. Catal Lett 28, 249–262 (1994). https://doi.org/10.1007/BF00806054
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00806054