Summary
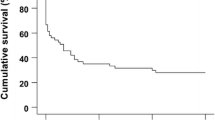
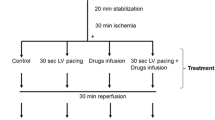
This study investigated whether increasing the magnesium concentration during secondary cardioplegia improves postischemic myocardial recovery. Twenty-four isolated pig hearts were divided into four groups. All hearts were initially subjected to control perfusion with modified Krebs-Henseleit solution for 30 min, followed by a single infusion of St. Thomas' solution #2. The hearts were then maintained without perfusion at 12°C for 4h. Following this hypothermic preservation, the hearts in group I were reperfused with modified Krebs-Henseleit solution for 50 min, while hearts in group II and III were reperfused with a secondary cardioplegic solution containing 16 or 0 mmol/L magnesium, respectively, for 20 min followed by 30 min of perfusion with modified Krebs-Henseleit solution. In group IV, the hearts were initially reperfused with Krebs-Henseleit solution containing 16 mmol/L potassium for 20 min, followed by 30 min of reperfusion with modified Krebs-Henseleit solution. The changes in high-energy phosphates and intracellular pH were monitored throughout the experiments using31P nuclear magnetic resonance (NMR) spectroscopy. Heart rate, left-ventricular systolic developed pressure, and rates of pressure increase and decrease were measured during control perfusion and reperfusion to calculate the percent contractile functional recovery. Needle biopsies for measurement of energy metabolites with high performance liquid chromatography were performed at the end of preservation and reperfusion to confirm the NMR measurements. All six hearts in group I showed significantly less recovery of contractile function during reperfusion when compared to the hearts in groups II, III, IV (p<0.05). There was no difference in either recovery of metabolism or mechanical function among the latter three groups of hearts. None of hearts in groups II, III, and IV showed ventricular fibrillation, which occurred in all six hearts of group I upon reperfusion. The results suggest that a short period of re-arrest perfusion following ischemia (“secondary cardioplegia”) improves postischemic contractile functional recovery and prevents reperfusion-induced ventricular fibrillation. Increased magnesium concentration in the secondary cardioplegia did not provide additional benefit to the ischemic myocardium, possibly due to the low permeability of the sarcolemmal membrane to magnesium.
Similar content being viewed by others
References
Hearse DJ, Bolli R (1991) Reperfusion-induced injury: Manifestations, mechanisms, and clinical relevance. Trends Cardiovasc Med 1:233–240
Ambrosio G, Chiariello M (1991) Myocardial reperfusion injury: Mechanisms and management —A review. Am J Med 91): (suppl 3C)86S-88S
Lazar HL, Buckberg GD, Manganaro AJ, Foglia RP, Becker H, Mulder DG, Maloney JV (1979) Reversal of ischemic damage with secondary blood cardioplegia. J Thorac Cardiovasc Surg 78:688–697
Acar C, Partington MT, Buckberg GD (1991) Studies of controlled reperfusion after ischemia. Reperfusate composition: Detrimental effects of initial asanguineous cardioplegic washout after acute coronary occlusion. J Thorac Cardiovasc Surg 101:294–302
Allen BS, Okamoto F, Buckberg GD, Acar C, Partington MT, Bugyi H, Leaf J (1986) Studies of controlled reperfusion after ischemia. Reperfusate composition: Benefits of marked hypocalcemia and diltiazem on regional recovery. J Thorac cardiovasc Surg 92:564–572
Okamoto F, Allen BS, Buckberg GD, Young H, Bugyi H, Leaf J (1986) Studies of controlled reperfusion after ischemia. Reperfusate composition: Interaction of marked hyperglycemia and marked hyperosmolarity in allowing immediate contractile recovery after four hours of regional ischemia. J Thorac Cardiovasc Surg 92:583–593
Follette DM, Fey K, Buckberg GD, Helly JJ, Steed DL, Foglia RP, Maloney JV (1981) Reducing postischemic damage by temporary modification of reperfusate calcium, potassium, pH, and osmolarity. J Thorac Cardiovasc Surg 82:221–238
Tian GH, Mainwood GW, Biro GP, Smith KE, Butler KW, Lawrence D, Deslauriers R (1991) The effect of high buffer cardioplegia and secondary cardioplegia on cardiac preservation and postischemic functional recovery: A31P NMR and functional study in Langendorff perfused pig hearts. Can J Physiol Pharmacol 69:1760–1768
Shine KI, Douglas AM (1983) Low calcium reperfusion of ischemic myocardium. J Mol Cell Cardiol 15:251–260
Ruigrok TJC, Kirkels JH, Schreur JHM, Van Echteld CJA (1991) A phosphorus-31 nuclear magnetic resonance study of myocardial ATP content during postischemic low calcium reperfusion. Bratisl Lek Listy 92:119–123
Bersohn MM, Shine KI, Sterman WD (1982) Effect of increased magnesium on recovery from ischemia in rat and rabbit hearts. Am J Physiol 242 (Heart Circ Physiol 11): H89-H93
Singh RB, Sircar AR, Rastogi SS, Garg V (1990) Magnesium and potassium administration in acute myocardial infarction. Magnesium Trace Elem 9:198–204
White RE, Hartzell HC (1988) Effects of intracellular free magnesium on calcium current in isolated cardiac myocytes. Science 239:778–780
Horie M, Irisawa H (1987) Rectification of muscarinic K+ current by magnesium ion in guinea pig atrial cells. Am J Physiol 253:H210–214
Kirkels JH, Van Echteld CJA, Ruigrok TJC (1989) Intracellular magnesium during myocardial ischemia and reperfusion: Possible consequences for postischemic recovery. J Mol Cell Cardiol 21:1209–1218
Borchgrevink PC, Bergan AS, Bakøy OE, Jynge P (1989) Magnesium and reperfusion of ischemic rat heart as assessed by31P NMR. Am J Physiol 256:H195–204
Ferrari R, Albertini A, Curello S, Ceconi C, Di Lisa F, Raddino R, Visioli O (1986) Myocardial recovery during post-ischemic reperfusion: Effects of nifedipine, calcium and magnesium. J Mol Cell Cardiol 18:487–498
David G Gadian (1982) Nuclear magnetic resonance and its applications to living systems. Clarendon, Oxford pp 1–22
Pond WG (1986) Nutrition and the cardiovascular system of swine. In: Stanton HC, Mersmann HJ (ed) Swine in cardiovascular research 2∶1−32, CRC, Boca Raton, Florida, pp 1–32
Deslauriers R, Butler KW, Saunders JK, Lareau S, Kroft T, Scott JR, Forester G, Tian GH, Mainwood GW (1989) NMR studies of long-term hypothermic preservation of porcine hearts: Effect of slow perfusion on high energy phosphates and developed force. Bull Magn Reson 11:143–149
Murphy E (1991) Cellular magnesium and Na/Mg exchange in heart cells. Annu Rev Physiol 53:273–287
Hess P, Lansman JB, Tsien RW (1986) Calcium channel selectivity for divalent and monovalent actions. J Gen Physiol 88:293–318
Lansman P, Hess JB, Tsien RW (1986) Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. J Gen Physiol 88:321–347
Agus ZS, Morad M (1991) Modulation of cardiac ion channels by magnesium. Annu Rev Physiol 53:299–307
Ishihara K, Mitsuiye T, Noma A, Takano M (1989) The Mg2+ block and intrinsic gating underlying inward rectification of the K+ current in guinea pig cardiac myocytes. J Physiol 419:297–320
Matsuda H (1988) Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. J Physiol 397:237–258
Flatman PW (1991) Mechanisms of magnesium transport. Annu Rev Physiol 53:259–271
Flatman PW (1984) Magnesium transport across cell membranes. J Membr Biol 80:1–14
Flatman PW (1988) The control of red cell magnesium. Magnesium Res 1:5–11
Fry CH (1986) Measurement and control of intracellular magnesium ion concentration in guinea pig and ferret ventricular myocardium. Magnesium 5:306–331
Murphy E, Steenbergen C, Levy LA, Raju B, London RE (1989) Cytosolic free magnesium levels in ischemic rat heart. J Biol Chem 264:5622–5627
Gebhard MM, Bretschneider HJ, Schnabel PA (1989) Cardioplegia principles and problems. In: Sperelakis N (ed) Physiology and Pathophysiology of the Heart, 2nd edition. Kluwer Academic Publishers, Boston, pp 655–669
Beyenbach KW (1990) Transport of magnesium across biological membranes. Magnesium Trace Elem 9:233–254
Anlev E, Teisinger J, Svoboda P (1987) Mg2+-induced changes of lipid order and conformation of (Na+-K+)-ATPase. Biochem Biophys Acta 905:376–382
Prescott AR, Comerford JG, Magrath R, Lamb NJC, Warn RM (1988) Effects of elevated intracellular magnesium on cytoskeletal integrity. J Cell Sc 89:321–329
Author information
Authors and Affiliations
Additional information
Supported by the Heart and Stroke Foundation of Ontario
Rights and permissions
About this article
Cite this article
Tian, G., Biro, G.P., Xiang, B. et al. The effect of magnesium added to secondary cardioplegia on postischemic myocardial metabolism and contractile function —a31P NMR spectroscopy and functional study in the isolated pig heart. Basic Res Cardiol 87, 356–365 (1992). https://doi.org/10.1007/BF00796521
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00796521