Conclusions
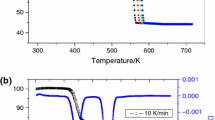
Use of the reducing hydrogen medium in thermal dissociation of nickel oxalate makes it possible to produce a nickel powder with a low content of oxides and a high specific surface. The optimum temperature conditions for producing a powder with the maximum specific surface are determined by the temperature of the start of sintering high-dispersion nickel. The powder has a developed fine crystalline structure whose specific surface is an order of magnitude larger than the inherent specific surface of the powder.
Similar content being viewed by others
Literature cited
New Process and Materials of Powder Metallurgy [in Russian], Metallurgiya, Moscow (1983).
I. A. Allen and D. E. Scaif, “The thermal decomposition of nickel oxalate,” J. Chem. Phys.,58, No. 8, 667–671 (1954).
R. W. Jacob and A. R. T. Kureishy, “The thermal decomposition of nickel oxalate,” Trans. Farad. Soc.,58, Part 3, 551–560 (1962).
J. Jach and M. Griffel, “The thermal decomposition of irradiated nickel oxalate,” J. Phys. Chem.,68, No. 5, 731–746 (1964).
D. Broadbent, D. Dollimore, and J. Dollimore, “The thermal decomposition of oxalates. V. The thermal decompsotion of nickel oxalate,” J. Chem. Soc. (A), No. 3 (1966).
K. Umemura, “Thermal dissociation of dihydrates of oxalates of nickel, cobalt, and iron in different atmospheres,” Nihon Kagai Kaisi, No. 6, 968–975 (1975).
V. V. Budkevich, T. A. Val'chuk, L. Yu. Ivanova, et al., “Examination of the process of reduction of multicomponent metallic magnetic powders,” Poroshk, Metall., No. 8, 1–5 (1976).
Yu. I. Kimchenko, V. P. Vasilenko, L. S. Radkevich, et al., “Process of decomposition of formiates of iron, cobalt, nickel, and copper,” Poroshk. Metall., No. 5, 7–13 (1977).
S. P. Chizhik, L. K. Grigor'eva, R. N. Kuklin, et al., “A magnetic method of inspecting the content of the metallic phase in the products of thermolysis of metallo-organic compounds,” in:Proceedings of 15th All-Union Scientific and Technical Conference on Powder Metallurgy, Institute of Problems of Materials Science, Academy of Sciences of the Ukrainian SSR, Kiev (1985), p. 268.
N. Henney, Chemistry of Solids [Russian translation], Mir, Moscow (1971).
S. P. Chizhik, L. K. Grigor'eva, and R. N. Kuklin, “Examination of the kinetics of sintering of powders of various dispersion,” Izv. Akad. Nauk SSSR, Met., No. 2 (1984).
Author information
Authors and Affiliations
Additional information
Translated from Poroshkovaya Metallurgiya, No. 1(325), pp. 57–61, January, 1990.
Rights and permissions
About this article
Cite this article
Gladkikh, N.T., Grigor'eva, L.K., Kuklin, R.N. et al. Kinetics of isothermal decomposition of nickel oxalate in hydrogen. Powder Metall Met Ceram 29, 51–55 (1990). https://doi.org/10.1007/BF00796094
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00796094