Abstract
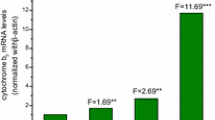
The activities of several different phase I and phase II drug-metabolizing enzymes were measured in freshly isolated oval cells from rats fed a choline-deficient/DL-ethionine-supplemented diet for 6 weeks and alsoin vitro in the established oval cell line OC/CDE 6. No cytochrome P450 was spectrophotometrically measurable in both preparations and two cytochrome P450-dependent monoxygenase activities, aminopyrineN-demethylase and ethoxyresorufinO-deethylase, could not be detected in the oval cells of both sources. However, cytosolic glutathione transferase, microsomal expoxide hydrolase and UDP-glucuronosyltransferase activities were clearly measurable in oval cells. Similar enzyme activities were found in freshly isolated and cultured oval cells. The highest activities of these three enzymes were detected during the exponential growth phase of the cultured cells; thereafter the activities decreased until the cells reached confluency. Changes in phenol UDP-glucuronosyltransferase (UGT1A1) mRNA levels paralleled the variations in UDP-glucuronosyltransferase activity, i.e. they were high in exponentially growing oval cells and low in confluent cell cultures. Taking into account that oval cells are able to proliferate in the livers of rats continuously fed a choline-deficient/DL-ethionine-supplemented diet and that none of the analyzed drug metabolizing enzymes are involved in the activation or detoxication ofDL-ethionine, the described pattern might be part of a more general, nonspecific, protection mechanism enabling these cells to overcome the cytotoxic effects of a variety of carcinogens and to proliferate even in their presence. Furthermore, the expression of microsomal epoxide hydrolase, cytosolic glutathione transferase and UDP-glucuronosyltransferase appears to depend on the proliferative status of the cells.
Similar content being viewed by others
Abbreviations
- CDE:
-
choline-deficient/DL-ethionine-supplemented diet
- GST:
-
glutathione transferase
- mEH:
-
microsomal epoxide hydrolase
- UGT:
-
UDP-glucuronosyltransferase
References
Bannasch P, Massner B. Histogenese und Cytogenese von Cholangiofibromen und Cholangiocarcinomen bei Nitrosomorpholinvergifteten Ratten. Z Krebsforsch. 1976; 87:239–55.
Bannasch P, Reiss W. Histogenese und Cytogenese cholangiocellulärer Tumoren bei Nitrosomorpholinvergifteten Ratten. Zugleich ein Beitrag zur Morphogenese der Cystenleber. Z Krebsforsch. 1971; 76:193–215.
Batist G, Woo A, Tsao M-S. Effect of proliferative state on glutathione S-transferase isoenzyme expression in cultured rat liver epithelial cells. Carcinogenesis. 1991; 12:2031–4.
Bock KW, Münzel PA, Röhrdanz E, Schrenk D, Eriksson LC. Persistently increased expression of a 3-methylcholanthrene-inducible phenol uridine diphosphateglucuronosyltransferase in rat hepatocyte nodules and hepatocellular carcinomas. Cancer Res. 1990; 50:3569–73.
Bock KW, Gschaidmaier H, Seidel A, Baird S, Burchell B. Monoand diglucuronide formation from chrysene and benzo(a)pyrene phenols by 3-methylcholanthrene-inducible phenol UDP-glucuronosyltransferase (UGT1A1). Mol Pharmacol. 1992; 42:613–8.
Burchell B, Nebert DW, Nelson DR et al. the UDP-glucuronosyltransferase gene superfamily: suggested nomenclature based on evolutionary divergence. DNA and Cell Biol. 1991; 10:487–94.
Burke MD, Mayer RT. Ethoxyresorufin: direct fluorimetric assay of a microsomalO-dealkylation which is preferentially inducible by 3-methylcholanthrene. Drug Metab Dispos. 1974; 2:583–8.
Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylaminofluorene and 3′-methyl-4-dimethylaminoazobenzene, Cancer Res. 1956; 16:142–9.
Fausto N, Thompson NL, Braun L. Purification and culture of oval cells from rat liver. In: Pretlow TG II, Pretlow TG, eds. Cell separations: methods and applications, vol. 4. New York: Academic Press; 1987:45–77.
Franson KL, Burchell B, Mathis GA. 17β-Hydroxysteroid UDP-glucuronosyltransferase is expressed in bile ductular epithelial cells under physiological conditions. Biochem Biophys Res Commun. 1990; 173:1001–7.
Glatt HR, Billings R, Platt KL, Oesch F. Improvement of the correlation of bacterial mutagenicity with carcinogenicity of benzo(a)pyrene and four of its major metabolites by activation with intact liver cells instead of cell homogenate. Cancer Res. 1981; 41:270–7.
Gottesman MM, Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988; 263:12163–6.
Grisham JW, Hartroft WS. Morphologic identification by electron microscopy of “oval cells” in experimental hepatic degeneration. Lab Invest. 1961; 10:317–32.
Grisham JW, Porta EA. Origin and fate of proliferated hepatic ductal cells in the rat: electron microscopic and autoradiographic studies. Exp Mol Pathol. 1964; 3:242–61.
Habig WH, Pabst MJ, Jakoby WB. Glutathione transferases: the first step in mercapturic acid formation. J Biol Chem. 1974; 249:7130–9.
Hacker HJ, Steinberg P, Toshkov I, Oesch F, Bannasch P. Persistence of the cholangiocellular and hepatocellular lesions observed in rats fed a choline-deficient/DL-ethionine-supplemented diet. Carcinogenesis. 1992; 13:271–6.
Jansen PLM, Mulder GJ, Burchell B, Bock KW. New developments in glucuronidation research: report of a workshop on “Glucuronidation, its role in health and disease”. Hepatology. 1992; 15:532–44.
Ledda GM, Sells MA, Yokoyama S, Lombardi B. Metabolic properties of isolated rat liver cell preparations enriched in epithelial cells other than hepatocytes. Int J Cancer. 1983; 31:231–7.
Lilienblum W, Walli, AK, Bock KW. Differential induction of rat liver microsomal UDP-glucuronosyltransferase activities by various inducing agents. Biochem Pharmacol. 1982; 31: 907–13.
Lowry OH, Rosebrough J, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–75.
Mathis GA, Walls S, D'Amico P, Gengo TF, Sirica A. Enzyme profile of rat bile ductular epithelial cells in reference to the resistance phenotype in hepatocarcinogenesis. Hepatology. 1989; 9:477–85.
Mazel P. The effect of phenobarbital pretreatment on microsomal cytochrome P-450 and cytochrome b5. In: La Du BN, Mandel HG, Way EL, eds. Fundamentals of drug metabolism and drug disposition. Baltimore: Williams & Wilkins; 1971:573–75.
Nakatsukasa H, Evarts RP, Burt RK, Nagy P, Thorgeirsson SS. Cellular pattern of multidrug-resistance gene expression during chemical hepatocarcinogenesis in the rat. Mol Carcinogenesis. 1992; 6:190–8.
Oesch F. Purification and specificity of a microsomal human epoxide hydrolase. Biochem J. 1974; 139:77–88.
Oesch F, Jerina DM, Daly J. A radiometric assay for hepatic epoxide hydrolase activity with [7-3H]styrene oxide. Biochim Biophys Acta. 1971; 227:685–91.
Omura T, Sato R. The carbon monoxide-binding protein of liver microsomes. J Biol Chem. 1964; 239:2370–85.
Pack R, Heck R, Dienes HP, Oesch F, Steinberg P. Isolation, biochemical characterization, long-term culture, and phenotype modulation of oval cells from carcinogen-fed rats. Exp Cell Res. 1993; 204:198–209
Rubin E. The origin and fate of proliferated bile ductular cells. Exp Mol Pathol. 1964; 3:279–86.
Sasaki T, Yoshida T. Experimentelle Erzeugung des Lebercarcinoms durch Fütterung mito-Amidoazotoluol. Virchows Arch Pathol Anat Physiol. 1935; 295:175–200.
Schrenk D, Eisenmann-Tappe I, Gebhardt R et al. Drug metabolizing enzyme activities in rat liver epithelial cell lines, hepatocytes and bile duct cells. Biochem Pharmacol. 1991; 41:1751–7.
Seglen PO. Preparation of rat liver cells. III. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973; 82:391–8.
Steinberg P, Hacker HJ, Dienes HP, Oesch F, Bannasch P. Enzyme histochemical and immunohistochemical characterization of oval and parenchymal cells proliferating in livers of rats fed a choline-deficient/DL-ethionine- supplemented diet. Carcinogenesis. 1991; 12:225–31.
Wilson JW, Leduc EH. Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol. 1958; 76:441–9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Steinberg, P., Steinbrecher, R., Schrenk, D. et al. Drug-metabolizing enzyme activities in freshly isolated oval cells and in an established oval cell line from carcinogen-fed rats. Cell Biol Toxicol 10, 59–65 (1994). https://doi.org/10.1007/BF00757187
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00757187