Abstract
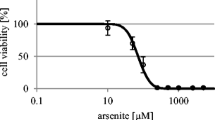
Our data show that a short incubation with arsenite (30–300 μM) induces a biphasic change in ceSlular sensitivity towards a second exposure to arsenite. A transient sensitization was followed by the development of self-tolerance. Sensitization was measured using the step-down protocol; i.e., application of a high dose of arsenite pretreatment (100 or 300 μM) followed immediately by incubation in a low dose of arsenite (1–30 μM), with extensive rinsing in between. Whereas no effect of 1 and 3 μM on cellular survival is observed without pretreatment, a large decrease in cell survival can be established when these low doses of arsenite are applied immediately after a 1 hr pretreatment with 100 or 300 arsenite.
According to the step-down protocol, a high dose of toxic compounds is applied and is followed by prolonged incubation in a lower concentration of the initial toxic compound. This might be a more accurate model for studying the effects of toxic insults on cells and organisms in the manner in which they occur in their natural environment. The level of tolerance was determined by a 1 hr test treatment with 300 pM arsenite applied at different times after pretreatment. Using this fractionated treatment protocol, it was established that tolerance increases with the increasing time intervals between the sodium arsenite treatments, during the 6 hr studied.
These observations suggest that sensitization gradually decreases, whereas tolerance develops. Furthermore, our data indicate that the condition of pretreatment determines the extent to which the early sensitivity increases, as well as the development of tolerance later on. A relatively high arsenite concentration leads to more sensitized cells, which are transformed into more tolerant cells in comparison with the effect of a lower arsenite concentration.
Similar content being viewed by others
References
BARRETT, J.C., LAMB, P.W., WANG, T.C., and LEE, T.C. (1989). “Mechanisms of arsenic-induced cell transformation.” Biol. Trace Element Res. 21: 421–429.
BURDON, R.H. (1986). “Heat shock and the heat shock proteins.” Biochem. J. 240: 313–324.
CERVERA, J. (1985). “Induction of self tolerance and enhanced stress protein synthesis in L-132 cells by cadmium chloride and by hyperthermia.” Cell Biol. Int. Rep. 9: 131–141.
DICKOMEY E., EICKHOFF, J., and JUNG, H. (1984). “Thermotolerance and thermosensitization in CHO and R1H cells: A comparative study.” Int. J. Radiat. Biol. 46: 181–192.
Hahn, G.M. and Li, G.C. (1990). “Thermotolerance, thermoresistance and thermosensitization.” In: Stress Proteins in Biology and Medicine (R.I. Morimoto, A. Tissières, and C. Georgopoulos, eds.). Cold Spring Harbor Laboratory Press. pp. 79–100.
HENLE, K.J., KARAMUZ, J.E., and LEEPER, D.B. (1978). “Induction of thermotolerance in Chinese hamster ovary cells by high (45°) or low (40°) hyperthermia.” Cancer Res. 38: 570–574.
HENLE, K.J. (1980). “Sensitization to hyperthermia below 43°C induced in Chinese hamster ovary cells by step-down heating.” J. Natl. Cancer Inst. 64: 1479–1483.
JUNG, H. (1982). “Interaction of thermotolerance and thermo-sensitization induced in CHO cells by combined hyperthermic treatment at 40°C and 43°C.” Radiation Research 91: 433–446.
JUNG, H. (1989). “Step-down heating of CHO cells at 37.5–39°C.” Int. J. Hyperthermia 5: 665–673.
LEE, K.J. and HAHN, G.M. (1988). “Abnormal proteins as the trigger for the induction of stress responses: heat, diamide, and sodium arsenite.” J. Cell. Physiol. 136: 411–420.
LEE, T.C., OSHIMURA, M., and BARRETT, J.C. (1985). “Comparison of arsenic-induced cell transformation, cytotoxicity, mutation and cytogenetic effects in Syrian hamster embryo cells in culture.” Carcinogenesis 6: 1421–1426.
LEE, T.C., WEI, M.L., CHANG, W.J., HO, I.C., LO, J.F., JAN, K.Y., and HUANG, H. (1989). “Elevation of glutathione levels and glutathione S-transferase activity in arsenic resistant Chinese Hamster Ovary cells.” In Vitro Cellular and Developm. Biol. 25: 442–448.
LI, G.C. and HAHN, G.M. (1980). “A proposed operational model of thermotolerance based on effects of nutrients and the initial treatment temperature.” Cancer Res. 40: 4501–4508.
LINDEGAARD, J.C. and OVERGAARD, J. (1990). “Step-down heating in a C3H mammary carcinoma in vivo: effects of varying the time and temperature of the sensitizing treatment.” Int. J. Hyperthermia 6: 607–617.
MOOIBROEK, J., DIKOMEY, E., ZYWIETZ, F., and JUNG, H. (1988). “Thermotolerance kinetics and growth rate changes in the R1H tumour heated at 43°C.” Int. J. Hyperthermia 4: 677–686.
NOVER, L. (1984). Heat Shock Response of Eukaryotic cells. Springer Verlag, Berlin.
NOVER, L. (1991). Heat Shock Response (L. Nover, ed.). CRC Press, Boca Raton, FL.
SCHAMHART, D.H.J., VAN WALRAVEN, H.S., WIEGANT, F.A.C., LINNEMANS, W.A.M., VAN RIJN, J., VAN DEN BERG, J., and VAN WIJK, R. (1984). “Thermotolerance in cultured hepatoma cells: cell viability cell morphology, protein synthesis and heat-shock proteins.” Radiation Research 98: 82–95.
SCHAMHART, D.H.J., ZOUTEWELLE, G., VAN AKEN, J., and VAN WIJK, R. (1992). “Effects on the expression of heat shock proteins by step-down heating and hyperthermia in rat hepatoma cells with a different degree of heat sensitivity.” Int. J. Hyperthermia 8: 701–716.
SQUIBB, K.S. and FOWLER, B.A. (1983). “Toxicity of arsenic and its compounds.” In: Biological and Environmental Effects of Arsenic. (B.A. Fowler, ed.). Elsevier Science Publishers, Amsterdam. pp. 234–269.
VASKEN APOSHIAN, H. (1989). “Biochemical toxicology of arsenic.” In: Reviews in Biochemical Toxicology. (E. Hodgson, J.R. Bend, and R.M. Philpot, eds.). Vol 10. Elsevier, New York. pp. 265–299.
Welch, W.J. (1990). “The mammalian stress response: cell physiology and biochemistry of stress proteins.” In: Stress Proteins in Biology and Medicine (R.I. Morimoto, A. Tissières, and C. Georgopoulos, eds.). Cold Spring Harbor Laboratory Press. pp. 223–278.
WIEGANT, F.A.C., TUYL, M. and LINNEMANS, W.A.M. (1985). “Calmodulin-inhibitors prevent heat-induced cytoskeletal reorganization and potentiate hyperthermic cell killing.” Int. J. Hyperthermia 1: 157–169.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wiegant, F.A.C., Souren, J.E.M., Van Rijn, H. et al. Arsenite induced sensitization and self-tolerance of Reuber H35 hepatoma cells. Cell Biol Toxicol 9, 49–59 (1993). https://doi.org/10.1007/BF00755139
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00755139