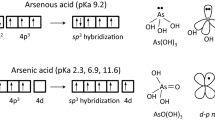
Abstract
Arsenic, a common poison, is known to react with sulfide in vivo, forming thioarsenates. The acute toxicity of the inorganic thioarsenates is currently unknown. Our experiments showed that a fourfold sulfide excess reduced acute arsenite cytotoxicity in human hepatocytes (HepG2) and urothelial cells (UROtsa) significantly, but had little effect on arsenate toxicity. Speciation analysis showed immediate formation of thioarsenates (up to 73 % of total arsenic) in case of arsenite, but no speciation changes for arsenate. Testing acute toxicity of mono- and trithioarsenate individually, both thioarsenates were found to be more toxic than their structural analogue arsenate, but less toxic than arsenite. Toxicity increased with the number of thio groups. The amount of cellular arsenic uptake after 24 h corresponded to the order of toxicity of the four compounds tested. The dominant to almost exclusive intracellular arsenic species was arsenite. The results imply that thiolation is a detoxification process for arsenite in sulfidic milieus. The mechanism could either be that thioarsenates regulate the amount of free arsenite available for cellular uptake without entering the cells themselves, or, based on their chemical similarity to arsenate, they could be taken up by similar transporters and reduced rapidly intracellularly to arsenite.






Similar content being viewed by others
References
Beak DG, Wilkin RT, Ford RG, Kelly SD (2008) Examination of arsenic speciation in sulfidic solutions using X-ray absorption spectroscopy. Environ Sci Technol 42(5):1643–1650
Burton ED, Johnston SG, Planer-Friedrich B (2013) Coupling of arsenic mobility to sulfur transformations during microbial sulfate reduction in the presence and absence of humic acid. Chem Geol 343:12–24. doi:10.1016/j.chemgeo.2013.02.005
Caro AA, Thompson S, Tackett J (2011) Increased oxidative stress and cytotoxicity by hydrogen sulfide in HepG2 cells overexpressing cytochrome P450 2E1. Cell Biol Toxicol 27(6):439–453. doi:10.1007/s10565-011-9198-2
Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ (1994) Reduction and binding of arsenate and dimethylarsinate by glutathione—a magnetic-resonance study. Chem-Biol Interact 90(2):139–155. doi:10.1016/0009-2797(94)90099-x
Dopp E, Hartmann LM, von Recklinghausen U, Florea AM, Rabieh S, Zimmermann U, Shokouhi B, Yadav S, Hirner AV, Rettenmeier AW (2005) Forced uptake of trivalent and pentavalent methylated and inorganic arsenic and its cyto-/genotoxicity in fibroblasts and hepatoma cells. Toxicol Sci 87(1):46–56. doi:10.1093/toxsci/kfi218
Dopp E, von Recklinghausen U, Hartmann LM, Stueckradt I, Pollok I, Rabieh S, Hao L, Nussler A, Katier C, Hirner AV, Rettenmeier AW (2008) Subcellular distribution of inorganic and methylated arsenic compounds in human urothelial cells and human hepatocytes. Drug Metab Dispos 36(5):971–979. doi:10.1124/dmd.107.019034
Eblin KE, Bowen ME, Cromey DW, Bredfeldt TG, Mash EA, Lau SS, Gandolfi AJ (2006) Arsenite and monomethylarsonous acid generate oxidative stress response in human bladder cell culture. Toxicol Appl Pharmacol 217(1):7–14. doi:10.1016/j.taap.2006.07.004
Fan HN, Wang HJ, Yang-Dan CR, Ren L, Wang C, Li YF, Deng Y (2013) Protective effects of hydrogen sulfide on oxidative stress and fibrosis in hepatic stellate cells. Mol Med Rep 7(1):247–253. doi:10.3892/mmr.2012.1153
Härtig C, Planer-Friedrich B (2012) Thioarsenate transformation by filamentous microbial mats thriving in an alkaline, sulfidic hot spring. Environ Sci Technol 46(8):4348–4356. doi:10.1021/es204277j
Hippler J, Zdrenka R, Reichel RAD, Weber DG, Rozynek P, Johnen G, Dopp E, Hirner AV (2011) Intracellular, time-resolved speciation and quantification of arsenic compounds in human urothelial and hepatoma cells. J Anal At Spectrom 26(12):2396–2403. doi:10.1039/c1ja10150a
Johnston SG, Burton ED, Keene AF, Planer-Friedrich B, Voegelin A, Blackford MG, Lumpkin GR (2012) Arsenic mobilization and iron transformations during sulfidization of As(V)-bearing jarosite. Chem Geol 334:9–24. doi:10.1016/j.chemgeo.2012.09.045
Jorgensen J, Mortensen PB (2001) Hydrogen sulfide and colonic epithelial metabolism—implications for ulcerative colitis. Dig Dis Sci 46(8):1722–1732. doi:10.1023/a:1010661706385
Kimura Y, Dargusch R, Schubert D, Kimura H (2006) Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signaling 8(3–4):661–670. doi:10.1089/ars.2006.8.661
Kimura Y, Y-i G, Kimura H (2011) Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. J Pharmacol Sci 115:77P–77P
Kimura Y, Y-i G, Kimura H (2012) Hydrogen sulfide prevents neuronal cell death by increasing glutathione production and suppressing oxidative stress in mitochondria. J Pharmacol Sci 118:104P–104P
Lerman SA, Clarkson TW, Gerson RJ (1983) Arsenic uptake and metabolism by liver-cells is dependent on arsenic oxidation-state. Chem-Biol Interact 45(3):401–406. doi:10.1016/0009-2797(83)90087-x
Liu ZJ, Carbrey JM, Agre P, Rosen BP (2004) Arsenic trioxide uptake by human and rat aquaglyceroporins. Biochem Biophys Res Commun 316(4):1178–1185. doi:10.1016/j.bbrc.2004.03.003
Naranmandura H, Ibata K, Suzuki KT (2007a) Toxicity of dimethylmonothioarsinic acid toward human epidermoid carcinoma A431 cells. Chem Res Toxicol 20(8):1120–1125. doi:10.1021/tx700103y
Naranmandura H, Suzuki N, Iwata K, Hirano S, Suzuki KT (2007b) Arsenic metabolism and thioarsenicals in hamsters and rats. Chem Res Toxicol 20(4):616–624. doi:10.1021/tx700038x
Naranmandura H, Ogra Y, Iwata K, Lee J, Suzuki KT, Weinfeld M, Le XC (2009) Evidence for toxicity differences between inorganic arsenite and thioarsenicals in human bladder cancer cells. Toxicol Appl Pharmacol 238(2):133–140. doi:10.1016/j.taap.2009.05.006
Naranmandura H, Carew MW, Xu S, Lee J, Leslie EM, Weinfeld M, Le XC (2011) Comparative toxicity of arsenic metabolites in human bladder cancer EJ-1 cells. Chem Res Toxicol 24(9):1586–1596. doi:10.1021/tx200291p
Nascimento MG, Suzuki S, Wei M, Tiwari A, Arnold LL, Lu X, Le XC, Cohen SM (2008) Cytotoxicity of combinations of arsenicals on rat urinary bladder urothelial cells in vitro. Toxicology 249(1):69–74. doi:10.1016/j.tox.2008.04.007
Ochi T, Kita K, Suzuki T, Rumpler A, Goessler W, Francesconi KA (2008) Cytotoxic, genotoxic and cell-cycle disruptive effects of thio-dimethylarsinate in cultured human cells and the role of glutathione. Toxicol Appl Pharmacol 228(1):59–67. doi:10.1016/j.taap.2007.11.023
Pinyayev TS, Kohan MJ, Herbin-Davis K, Creed JT, Thomas DJ (2011) Preabsorptive metabolism of sodium arsenate by anaerobic microbiota of mouse cecum forms a variety of methylated and thiolated arsenicals. Chem Res Toxicol 24(4):475–477. doi:10.1021/tx200040w
Planer-Friedrich B, Wallschlaeger D (2009) A critical investigation of hydride generation-based arsenic speciation in sulfidic waters. Environ Sci Technol 43(13):5007–5013. doi:10.1021/es900111z
Planer-Friedrich B, London J, McCleskey RB, Nordstrom DK, Wallschläger D (2007) Thioarsenates in geothermal waters of Yellowstone National Park: determination, preservation, and geochemical role. Environ Sci Technol 41(15):5245–5251
Planer-Friedrich B, Franke D, Merkel B, Wallschlager D (2008) Acute toxicity of thioarsenates to Vibrio fischeri. Environ Toxicol Chem 27(10):2027–2035
Planer-Friedrich B, Fisher JC, Hollibaugh JT, Süß E, Wallschläger D (2009) Oxidative transformation of trithioarsenate along alkaline geothermal drainages—abiotic versus microbially mediated processes. Geomicrobiol J 26:339–350
Planer-Friedrich B, Suess E, Scheinost AC, Wallschlaeger D (2010) Arsenic speciation in sulfidic waters: reconciling contradictory spectroscopic and chromatographic evidence. Anal Chem 82:10228–10235. doi:10.1021/ac1024717
Qu W, Liu J, Dill AL, Saavedra JE, Keefer LK, Waalkes MP (2009) V-PROLI/NO, a nitric oxide donor prodrug, protects liver cells from arsenic-induced toxicity. Cancer Sci 100(3):382–388. doi:10.1111/j.1349-7006.2008.01050.x
Radabaugh TR, Aposhian HV (2000) Enzymatic reduction of arsenic compounds in mammalian systems: reduction of arsenate to arsenite by human liver arsenate reductase. Chem Res Toxicol 13(1):26–30
Rader KJ, Dombrowski PM, Farley KJ, Mahony JD, Di Toro DM (2004) Effect of thioarsenite formation on arsenic(III) toxicity. Environ Toxicol Chem 23(7):1649–1654. doi:10.1897/03-443
Raml R, Rumpler A, Goessler W, Vahter M, Li L, Ochi T, Francesconi KA (2007) Thio-dimethylarsinate is a common metabolite in urine samples from arsenic-exposed women in Bangladesh. Toxicol Appl Pharmacol 222(3):374–380. doi:10.1016/j.taap.2006.12.014
Rochette EA, Bostick BC, Li GC, Fendorf S (2000) Kinetics of arsenate reduction by dissolved sulfide. Environ Sci Technol 34(22):4714–4720. doi:10.1021/es000963y
Schwedt G, Rieckhoff M (1996) Separation of thio- and oxothioarsenates by capillary zone electrophoresis and ion chromatography. J Chromatogr A 736(1–2):341–350. doi:10.1016/0021-9673(95)01319-9
Scott N, Hatlelid KM, Mackenzie NE, Carter DE (1993) Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem Res Toxicol 6(1):102–106. doi:10.1021/tx00031a016
Shinkai Y, Sumi D, Toyama T, Kaji T, Kumagai Y (2009) Role of aquaporin 9 in cellular accumulation of arsenic and its cytotoxicity in primary mouse hepatocytes. Toxicol Appl Pharmacol 237(2):232–236. doi:10.1016/j.taap.2009.03.014
Smith KR, Klei LR, Barchowsky A (2001) Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. Am J Physiol-Lung Cell Mol Physiol 280(3):L442–L449
Stauder S, Raue B, Sacher F (2005) Thioarsenates in sulfidic waters. Environ Sci Technol 39(16):5933–5939
Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ (2000) Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol 74(6):289–299
Suess E, Planer-Friedrich B (2012) Thioarsenate formation upon dissolution of orpiment and arsenopyrite. Chemosphere 89(11):1390–1398. doi:10.1016/j.chemosphere.2012.05.109
Suess E, Scheinost AC, Bostick BC, Merkel BJ, Wallschlaeger D, Planer-Friedrich B (2009) Discrimination of thioarsenites and thioarsenates by X-ray absorption spectroscopy. Anal Chem 81(20):8318–8326. doi:10.1021/ac901094b
Suess E, Wallschlaeger D, Planer-Friedrich B (2011) Stabilization of thioarsenates in iron-rich waters. Chemosphere 83:1524–1531
Thilo E, Hertzog K (1970) Processes in formation of As(V) sulfide in acidification of tetrathioarsenate solutions. Z Anorg Allg Chem 373(2):111. doi:10.1002/zaac.19703730203
Van de Wiele T, Gallawa CM, Kubachka KM, Creed JT, Basta N, Dayton EA, Whitacre S, Du Laing G, Bradham K (2010) Arsenic metabolism by human gut microbiota upon in vitro digestion of contaminated soils. Environ Health Perspect 118(7):1004–1009. doi:10.1289/ehp.0901794
Villa-Bellosta R, Sorribas V (2010) Arsenate transport by sodium/phosphate cotransporter type IIb. Toxicol Appl Pharmacol 247(1):36–40. doi:10.1016/j.taap.2010.05.012
Wallschläger D, Stadey CJ (2007) Determination of (oxy)thioarsenates in sulfidic waters. Anal Chem 79:3873–3880
Wen Y-D, Wang H, Kho S-H, Rinkiko S, Sheng X, Shen H-M, Zhu Y-Z (2013) Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS ONE 8(2):e53147. doi:10.1371/journal.pone.0053147
Wilkin R, Wallschläger D, Ford R (2003) Speciation of arsenic in sulfidic waters. Geochem T 4(1):1–7
Xiao LC, Lan AP, Mo LQ, Xu WM, Jiang N, Hu F, Feng JQ, Zhang CR (2012) Hydrogen sulfide protects PC12 cells against reactive oxygen species and extracellular signal-regulated kinase 1/2-mediated downregulation of glutamate transporter-1 expression induced by chemical hypoxia. Int J Mol Med 30(5):1126–1132. doi:10.3892/ijmm.2012.1090
Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, Masuko T, Nishikawa H, Kawabata A (2007) A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology 241(1–2):11–18. doi:10.1016/j.tox.2007.07.020
Acknowledgments
We acknowledge generous funding by the German Research Foundation within the Emmy Noether program to B. Planer-Friedrich (grant # PL 302/3-1) and thank Stefan Will for help with AEC-ICP-MS analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Data about instrumental parameters for the separation of arsenic species by anion exchange chromatography is available in Online Resource Table SI-1; arsenic speciation data (intracellular and in cell growth medium) after 24 h exposure to the four investigated arsenic compounds are available in Online Resource Figures SI-1 and SI-2.
ESM 1
(PDF 99 kb)
Rights and permissions
About this article
Cite this article
Hinrichsen, S., Lohmayer, R., Zdrenka, R. et al. Effect of sulfide on the cytotoxicity of arsenite and arsenate in human hepatocytes (HepG2) and human urothelial cells (UROtsa). Environ Sci Pollut Res 21, 10151–10162 (2014). https://doi.org/10.1007/s11356-014-2950-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2950-4