Abstract
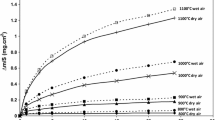
Kinetics of oxidation of Co-Cr alloys containing 0.4%–15% Cr was studied as a function of temperature (1273–1573 K) and oxygen pressure (4 × 102–105Pa). The oxidation process was found to be approximately parabolic and faster than that for pure cobalt. The scales are double-layered and consist of a compact outer CoO layer and a porous inner layer containing CoO slightly doped by chromium and spinel CoCr2O4. The oxidation mechanism was investigated by means of platinum markers and the18O isotope. The scale on the alloys containing less than 1% Cr grows exclusively by outward diffusion of cobalt, while that on the alloys containing more chromium—with a significant contribution of inward oxygen transport from atmosphere. This transport is not a lattice diffusion, but proceeds presumably through microfissures resulting from the secondary process of perforating dissociation of the outer scale layer.
Similar content being viewed by others
References
F. R. Billman,J. Electrochem. Soc. 119, 1198 (1972).
P. K. Kofstad and A. Z. Hed,J. Electrochem. Soc. 116, 224; 229; 1542 (1969).
P. K. Kofstad and A. Z. Hed,Werkst. Korros. 21, 895 (1970).
I. G. Wright and G. C. Wood,Oxid. Met. 11, 163 (1977).
A. Davin, D. Coutsouradis, and L. Habraken,Cobalt,35, 69 (1967).
S. N. Sumin and A. S. Tumariev,Izv. Vyssh. Uchebn. Zved. Tsvet. Met. 5, 34 (1967).
F. H. Stott, I. G. Wright, T. Hodgkiess, and G. C. Wood,Oxid. Met. 11, 141 (1977).
K. Przybylski and D. Szwagierczak,Ann. Chim. Fr. 4, 61 (1979).
S. Mrowec, M. Lasoń, E. Fryt, K. Przybylski, and A. Ciembroniewicz,J. Thermal Anal. 6, 193 (1974).
S. Mrowec and K. Przybylski,Oxid. Met. 11, 365 (1977).
S. Mrowec,Corros. Sci. 7, 563 (1967).
R. Emmerich, S. Mrowec, K. Przybylski, and D. Szwagierczak,Bull. Acad. Polon. Sci. Sér. Sci. Chim., to be published in (1982).
A. Brückman,Corros. Sci. 7, 51 (1967).
R. Sun,J. Chem. Phys. 28, 290 (1950).
A. Morkel and H. Schmalzried,Z. Phys. Chem. N. F. 32, 76 (1962).
J. B. Holt,Proc. Br. Ceram. Soc. 11, 157 (1967).
W. K. Chen and R. A. Jackson,J. Phys. Chem. Solids,30, 1309 (1969).
W. K. Chen, N. L. Peterson, and W. T. Reeves,Phys. Rev. 186, 887 (1969).
J. M. Wimmer, R. N. Blumenthal, and I. Bransky,J. Phys. Chem. Solids,36, 269 (1975).
N. G. Eror and J. B. Wagner, Jr.,J. Phys. Chem. Solids,29, 1597 (1968).
E. Fryt, S. Mrowec, and T. Walec,Oxid. Met. 7, 117 (1973).
B. Fisher and D. S. Tannhauser,J. Chem. Phys. 44, 1663 (1966).
A. Brückman, R. Emmerich, and S. Mrowec,Oxid. Met. 5, 137 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Przybylski, K., Szwagierczak, D. Kinetics and mechanism of high-temperature oxidation of dilute cobalt-chromium alloys. Oxid Met 17, 267–295 (1982). https://doi.org/10.1007/BF00738387
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00738387