Summary
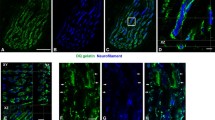
The distribution of three myofibrillar M-band proteins, myomesin, M-protein and the muscle isoform of creatine kinase, was investigated with immunocytochemical techniques in skeletal muscles of embryonic, fetal, newborn and four-week-old rats. Furthermore, muscles of newborn rats were denervated and examined at four weeks of age. In embryos, myomesin was present in all myotome muscle fibres of the somites, whereas M-protein was detected only in a small proportion of the myotome muscle fibres and muscle creatine kinase was not detected at all. In fetal and newborn muscles, all fibres contained all three M-band proteins. At four weeks of age, when fibre types (type 1 or slow twitch fibres and type 2 or fast twitch fibres) were clearly discernable, the pattern was changed. Myomesin and muscle creatine kinase were still observed in all fibres, whereas M-protein was present only in type 2 fibres. On the other hand, in muscle fibres denervated at birth all three M-band proteins were still detected. Our results suggest 1) that during the initial stages of myofibrillogenesis expression and incorporation of myomesin into the M-band precede that of M-protein and muscle creatine kinase; 2) that expression and incorporation of all three M-band proteins during fetal development is nerve independent and non coordinated to the expression of different forms of myosin heavy chains, and 3) that the suppression of M-protein synthesis during postnatal development is nerve dependent and reflects the maturation of slow twitch motor units.
Similar content being viewed by others
References
Arnold H, Pette D (1970) Binding of aldolase and TDA to F-actin and modificating of catalytic properties of aldolase. Eur J Biochem 15:360–366
Bergh A, Helander HF, Wahlqvist L (1978) Studies on factors governing testicular descent in the rat — particularly the role of gubernaculum testis. Int J Androl 1:342–356
Brooke MH, Kaiser KK (1970) Muscle fiber type: how many and what kind? Arch Neurol 23:369–379
Brown MC, Booth CM (1983) Postnatal development of the adult pattern of motor axon distribution in rat muscle. Nature 304:741–742
Butler-Browne GS, Whalen RG (1984) Myosin isozyme transitions occurring during the postnatal development of the rat soleus muscle. Dev Biol 102:324–334
Bähler M, Wallimann T, Eppenberger HM (1985) Myofibrillar M-band proteins represent constituents of native thick filaments, frayed filaments and bare zone assemblages. J Muscle Res Cell Motil 6:783–800
Carlsson E, Thornell L-E (1987) Diversification of the myofibrillar M-band in rat skeletal muscle during postnatal development. Cell Tissue Res 248:169–180
Close RI (1964) Dynamic properties of fast and slow skeletal muscles of the rat during development. J Physiol 173:74–95
Close RI (1972) Dynamic properties of mammalian skeletal muscle. Physiol Rev 52:129–197
Dhoot GK (1986) Selective synthesis and degradation of slow skeletal muscle myosin heavy chains in developing muscle fibers. Muscle Nerve 9:155–164
Drachman DB, Johnson DM (1973) Development of a mammalian fast muscle: dynamic and biochemical properties correlated. J Physiol 234:29–42
Eppenberger HM, Perriard JC, Rosenberg UB, Strehler EE (1981) The Mr 165000 M-protein myomesin. A specific protein of cross-striated muscle cell. J Cell Biol 89:185–193
Fairfield J (1948) Effects of cold on infant rats: body temperature, oxygen consumption, electrocardiograms. Am J Physiol 128:514
Gambke B, Rubinstein NA (1984) A monoclonal antibody to the embryonic myosin heavy chain of rat skeletal muscle. J Biol Chem 259:12092–12100
Gorza L (1990) Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and antimyosin monoclonal antibodies. J Histochem Cytochem 38:257–265
Grove BK, Kurer V, Lehner C, Doetschman TC, Perriard JC, Eppenberger HM (1984) A new 185000-dalton skeletal muscle protein detected by monoclonal antibodies. J Cell Biol 98:518–524
Grove BK, Cerny L, Perriard J-C, Eppenberger HM (1985) Myomesin and M-protein: expression of two M-band proteins in pectoral muscle and heart during development. J Cell Biol 101:1413–1421
Grove BK (1989) Muscle differentiation and the origin of muscle fiber diversity. CRC Crit Rev Neurobiol 4:201–233
Grove BK, Cerny L, Perriard J-C, Eppenberger HM, Thornell L-E (1989) Fiber type-specific distribution of M-band proteins in chicken muscle. J Histochem Cytochem 37:447–454
Knappeis GG, Carlsen F (1968) The ultrastructure of the M-line in skeletal muscle. J Cell Biol 38:202–211
Kugelberg E (1976) Adaptive transformation of rat soleus motor units during growth. Histochemistry and contraction speed. J Neurol Sci 27:269–289
Luther P, Squire J (1978) Three-dimensional structure of the vertebrate muscle M-region. J Mol Biol 125:313–324
Lyons GE, Haselgrove J, Kelly AM, Rubenstein NA (1983) Myosin transitions in developing fast and slow muscles of the rat hindlimb. Differentiation 25:168–175
Masaki T, Takaiti O (1974) M-protein. J Biochem (Tokyo) 75:367–380
Narusawa M, Fitzsimmons RB, Jzumo S, Nadal-Girard B, Rubinstein NA, Kelly AM (1987) Slow myosin in developing rat skeletal muscle. J Cell Biol 104:447–459
Otsu N, Hirata M, Tuboi S, Miyazawa K (1989) Immunocytochemical localization of creatine kinase M in canine myocardial cells: most creatine kinase M is distributed in the A-band. J Histochem Cytochem 37:1465–1470
Riley DA (1977) Multiple innervation of fiber types in the soleus muscles of postnatal rats. Exp Neurol 56:400–409
Savabi F, Geiger PJ, Bessman SP (1983) Kinetic properties and functional role of creatine phosphokinase in glycerinated muscle fibers. Further evidence for compartmentation. Biochem Biophys Res Commun 114:785–790
Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lømo T (1989) Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil 10:197–205
Sjöström M, Thornell L-E, Cedergren E (1973) The application of cryoultramicroscopy in the study of the fine structure of myofilaments. J Microsc 99:193–204
Strehler EE, Carlsson E, Eppenberger HM, Thornell L-E (1983) Ultrastructural localization of M-band proteins in chicken breast muscle as revealed by combined immunocytochemistry and ultramicrotomy. J Mol Biol 166:141–158
Termin A, Staron RS, Pette D (1989) Myosin heavy chain isoforms in histochemically defined fiber types of rat musclce. Histochemistry 92:453–457
Thornell L-E, Butler-Browne GS, Carlsson E, Eppenberger HM, Fürst DO, Grove BK, Holmbom B, Small JV (1986) Cryoultramicrotomy and immunocyto-chemistry in the analysis of muscle fine structure. Scan Electron Microsc 9:1407–1418
Thornell L-E, Carlsson E, Kugelberg E, Grove BK (1987) Myofibrillar M-band structure and composition of physiologically defined rat motor units. Am J Physiol 253:456–468
Thornell L-E, Carlsson E, Pedrosa F (1990) M-band structure and composition in relation to fiber types. In: Pette D (ed) The dynamic state of muscle fibers. Walter de Gruyte, Berlin New York, pp 369–383
Tokuyasu KT (1980) Immunochemistry on ultrathin frozen sections. Histochem J 12:381–403
Turner DC, Wallimann T, Eppenberger HM (1973) A protein that binds specifically to the M-line of skeletal muscle is identified as the muscle form of creatine kinase. Proc Natl Acad Sci USA 70:702–705
Wallimann T, Turner DC, Eppenberger HM (1975) Creatine kinase and M-line structure. In: Biro EVA (ed) Proteins of contractile systems. Proc 9th Eur Meeting, Budapest, Hungary 1974. FEBS 31:119–124
Wallimann T, Turner DC, Eppenberger HM (1977a) Localization of creatine kinase isoenzymes in myofibrils. I. Chicken skeletal muscle. J Cell Biol 75:297–317
Wallimann T, Kuhn HJ, Pelloni G, Turner DC, Eppenberger HM (1977b) Localization of creatine kinase isoenzymes in myofibrils. I. Chicken heart muscle. J Cell Biol 75:318–325
Wallimann T, Pelloni G, Turner DC, Eppenberger HM (1978) Monovalent antibodies against MM-creatine kinase remove the M-line from myofibrils. Proc Natl Acad Sci USA 75:4296–4300
Wallimann T, Doetschman TC, Eppenberger HM (1983) Novel staining pattern of skeletal muscle M-lines upon incubation with antibodies against MM-creatine kinase. J Cell Biol 96:1772–1779
Wallimann T, Eppenberger HM (1985) Localization and function of M-line-bound creatine kinase. M-band model and creatine phosphate shuttle. Cell Muscle Motil 6:239–285
Wallimann T, Schnyder T, Schlegel J, Wyss M, Wegmann G, Rossi A-M, Hemmer W, Eppenberger HM, Quest AFG (1989) Subcellular compartmentation of creatine kinase isoenzymes, regulation of CK and octameric structure of mitochondrial CK: important aspects of thephosphoryl-creatine circuit. In: Paul RJ, Elzinga G, Yamada K (eds) Progress in clinical and biological research. Muscle Energetics, vol. 315. Alan R. Liss, New York, pp 159–176
Wegman R, Kishino Y, Samannoudy FA (1974) Histochemical study on creatine kinase of developing and denervated rat muscle as compared with glycolytic and oxidative enzymes. Ann Histochem 19:227–238
Wegmann E, Eppenberger HM, Wallimann T (1987) In situ localization of creatine kinase (CK) in skeletal muscle. J Muscle Res Cell Motil 8:90
Woodhead JL, Lowey S (1982) Size and shape of skeletal bound M-protein. J Mol Biol 157:149–154
Ziter FA (1974) Creatine kinease in developing skeletal and cardiac muscle of the rat. Exp Neurol 43:539–546
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carlsson, E., Grove, B.K., Wallimann, T. et al. Myofibrillar M-band proteins in rat skeletal muscles during development. Histochemistry 95, 27–35 (1990). https://doi.org/10.1007/BF00737225
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00737225