Abstract
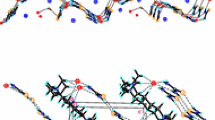
Tetrabenzo-18-crown-6 (1) shows distinct solid-state inclusion properties towards a number of OH-acidic, CH-acidic and low-polar uncharged organic molecules. The single crystal X-ray analysis of the inclusion complex between1 and EtNO2 (1∶1) is reported. Crystals are monoclinic,P21/c, witha=12.887(1),b=19.365(2),c=10.776(1) Å, β=96.33(2)o,D c=1.321g cm−1,Z=4. The host macroring has a conformation similar to a ‘dentist's-chair’. The complex is stabilized mainly by C−H...O type interactions involving the methyl group of the EtNO2 guest molecule which is highly disordered. The nitroethane guests are trapped in channels formed by the host macrocycles.
Similar content being viewed by others
References
C.J. Pedersen:Angew. Chem. 100, 1053 (1988);Angew. Chem., Int. Ed. Engl. 27, 1021 (1988).
Reviews: E. Weber: inSynthesis of Macrocycles: The Design of Selective Complexing Agents: Progress in Macrocyclic Chemistry, Vol. 3 (eds. R.M. Izatt and J.J. Christensen), p. 337, Wiley, New York (1987).
I. Goldberg: inCrown Ethers and Analogs (eds. S. Patai and Z. Rappoport), p. 399, Wiley, Chichester (1989).
More recent examples: G.W. Buchanan, C. Morat, J.P. Charland, C.I. Ratcliffe, and J.A. Ripmeester:Can. J. Chem. 67, 1212 (1989).
B. Belarmi, C. Bavoux, and A. Thozet:J. Incl. Phenom. 8, 383 (1990).
M. Perrin, E. Mahdar, S. Lecocq, and C. Bavoux:J. Incl. Phenom. 9, 153 (1990).
G.W. Gokel, D.J. Cram, C.L. Liotta, H.P. Harris, and F.L. Cook:J. Org. Chem. 39, 2445 (1974).
G.W. Gokel, D.J. Cram, C.L. Liotta, H.P. Harris, and F.L. Cook:Org. Synth. 57, 30 (1977).
F. Vögtle and W.M. Müller:Chem. Ber. 113, 2081 (1980).
F. Vögtle and W.M. Müller:Chem. Ber. 114 3179 (1981).
C.J. Pedersen:J. Org. Chem. 36, 1690 (1971).
A. van Zon, F. de Jong, D.N. Reinhoudt, G.J. Torny, and Y. Onwezen:Recl. Trav. Chim. Pay-Bas 100, 449 (1981).
R.D. Rogers:J. Incl. Phenom. 6, 629 (1988).
E. Weber:Chem. Ber. 118 4439 (1985).
E. Weber, S. Franken, J. Ahrendt, and H. Puff:J. Org. Chem. 52, 5291 (1987).
E. Weber, H.J. Köhler, and H. Reuter:Chem. Ber. 122, 959 (1989).
E. Weber, H.J. Köhler, K. Panneerselvam, and K.K. Chacko:J. Chem. Soc., Perkin Trans. 2 1599 (1990).
E. Weber, H.J. Köhler, and H. Reuter:J. Org. Chem. 56, 1236 (1991).
G.H. Stout and L.H. Jensen: inX-Ray Structure Determination. A Practical Guide, p. 454, MacMillan, New York (1968).
G.M. Sheldrick:SHELX 86. Program for the Solution of Crystal Structures from Diffractometer Data, University of Göttingen, Germany (1986).
G.M. Sheldrick:SHELX 76. Program for Crystal Structure Determination, University of Cambridge, England (1976).
D.T. Cromer and J.B. Mann:Acta Crystallogr., Sect. A. 24, 321 (1968).
R.F. Stewart, E.R. Davidson, and W.T. Simpson:J. Chem. Phys. 42, 3175 (1965).
E. Weber: inMolecular Inclusion and Molecular Recognition-Clathrates I; Topics in Current Chemistry, Vol. 140 (ed. E. Weber), p. 1, Springer-Verlag, Berlin-Heidelberg (1987).
E. Weber: inInclusion Compounds, Vol. 4 (eds. J.L. Atwood, J.E.D. Davies, and D.D. MacNicol), p. 188, Oxford University Press, Oxford (1991).
I. Csöregh, M. Czugler, E. Weber, and J. Ahrendt:J. Incl. Phenom. 8, 309 (1990).
E. Weber and H.P. Josel:J. Incl. Phenom. 1, 79 (1983).
E. Weber:J. Mol. Graphics 7, 12 (1989).
J.A.A. Boer, D.N. Reinhoudt, S. Harkema, G.J. van Hummel and F. de Jong:J. Am. Chem. Soc. 104, 4073 (1982).
R.D. Rogers and L.M. Green:J. Incl. Phenom. 4, 77 (1986).
R.D. Rogers and P.D. Richards:J. Incl. Phenom. 5, 631 (1987).
R.D. Rogers, L.K. Kurihara, and P.D. Richards:J. Chem. Soc., Chem. Commun., 604 (1987).
D. Bright and M.R. Truter:J. Chem. Soc. B., 1544 (1970).
W. Xu, K. Clinger, M.L. Hackert, and N.S. Poonia:J. Incl. Phenom. 3, 163 (1985).
P.J. Reddy, K.K. Chacko, I.H. Suh, E. Weber, H.J. Köhler, and M. Piel:Inorg. Chim. Acta 207, 31 (1993).
Tables of Interatomic Distances and Configurations in Molecules and Ions, The Chemical Society, London (1960).
G. Weber and P.G. Jones:Acta Crystallogr. Sect. C 39, 1577 (1983).
M. Dobler:Ionophores and their Structure, Wiley, New York (1981).
R. Hilgenfeld and W. Saenger:Top. Curr. Chem. 101, 1 (1982).
F. Vögtle, W.M. Müller, and W.H. Watson:Top. Curr. Chem. 125, 131 (1984).
M. Mercer and M.R. Truter:J. Chem. Soc., Dalton Trans., 2469 (1973).
D.L. Hughes, C.L. Mortimer and M.R. Truter:Inorg. Chim. Acta 29, 43 (1978).
R. Taylor and O. Kennard:J. Am. Chem. Soc. 104, 5063 (1982).
G.R. Desiraju:Acc. Chem. Res. 24, 290 (1991).
L. Pauling: inThe Nature of the Chemical Bond, 3rd. ed., p. 260, Cornell Universitiy Press, Ithaca (1960).
I. Goldberg:Acta Crystallogr. Sect. B. 31, 754 (1975).
R. Kaufmann, A. Knöchel, J. Kopf, J. Oehler, G. Rudolph:Chem. Ber. 110, 2249 (1977).
R. Gerdil: inMolecular Inclusion and Molecular Recognition-Clathrates I; Topics in Current Chemistry, Vol. 140 (ed. E. Weber), p. 71, Springer-Verlag, Berlin-Heidelberg (1987).
W.D. Ollis and J.F. Stoddart: inInclusion Compounds, Vol. 2 (eds. J.L. Atwood, J.E.D. Davies, and D.D. MacNicol), p. 169, Academic Press, London (1984).
E. Giglio: inInclusion Compounds, Vol. 2 (eds. J.L. Atwood, J.E.D. Davies, and D.D. MacNicol), p. 207, Academic Press, London (1984).
R.E. Koeppe, K.O. Hodgson, and L. Stryer:J. Mol. Biol. 121, 41 (1978).
R.E. Koeppe, K.O. Hodgson, and L. Stryer:Nature 279, 723 (1979).
O. Heitzsch, K. Gloe, A. Sabela, J. Koryta, and E. Weber:J. Incl. Phenom. 13, 311 (1992).
Author information
Authors and Affiliations
Additional information
Supplementary Data relating to this article are deposited with the British Library as Supplementary Publication No. SUP 82150 (11 pages)
Rights and permissions
About this article
Cite this article
Weber, E., Ahrendt, J., Lohner, A. et al. Crystalline inclusion compounds of tetrabenzo-18-crown-6 with uncharged organic molecules. Solid-state structure of the nitroethane complex. J Incl Phenom Macrocycl Chem 15, 231–245 (1994). https://doi.org/10.1007/BF00709069
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00709069