Abstract
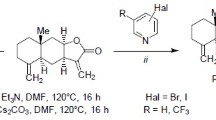
Electrophilic cyclization of (cyclo)farnesanes containing anexo-methylene group in the a-isoprenoid unit smoothly gives regio- and stereoisomeric octalins subsequently transformed to tricyclic furanosesquiterpenoids related to metabolites of some marine organisms.
Similar content being viewed by others
References
A. A. Surkova, A. V. Lozanova, and A. M. Moiseenkov,Izv. Akad. Nauk, Ser Khim., 1992, 471 [Bull. Acad. Sci.,Div. Chem. Sci., 1992,41, 376 (Engl. Transl.)].
G. Cimino, S. de Stefano, A. Guerriero, and L. Minale,Tetrahedron Lett., 1975, (a) 1421, (b) 1425.
R. P. Walker, R. M. Rosser, and D. J. Faulkner,J. Org. Chem., 1984,49, 5160.
D. Nasipuri and G. Das,J. Chem. Soc., Perkin Trans, 1, 1979, 2776.
S. P. Tanis and P. M. Herrinton,J. Org. Chem., 1983,48, 4572.
A. B. Smith III and R. Mewshaw,J. Org. Chem., 1984,49, 3685.
D. Liotta and W. Ott,Synth. Commun., 1987,17, 1655.
K. Shishido, K. Umimoto, and M. Shibuya,Heterocydes, 1990,31, 597.
T. Matsumoto and S. Usui,Chem. Lett., 1978, 105.
H. Akita and T. Oishi,Chem. Pharm. Bull., 1981,29, 1580.
L. Poppe, L. Novak, and C. Szantay,Synth. Commun., 1987,17, 173.
Y. Yamamura, K. Umeyama, K. Maruoka, and H. Yamamoto,Tetrahedron Lett, 1982,23, 1933.
V. A. Smit, A. V. Semenovskii, B. A. Rudenko, and V. F. Kucherov,Izv. Akad. Nauk SSSR, Ser. Khim., 1963, 1782 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1963,12 (Engl. Transl.)].
A. V. Semenovskii, V. A. Smit, and V. F. Kucherov,Dokl. Akad. Nauk SSSR, 1965,160, 1097 [Dokl. Chem., 1965,160 (Engl. Transl.)].
A. V. Semenovskii, V. A. Smit, and V. F. Kucherov,Izv. Akad. Nauk SSSR, Ser. Khim., 1965, 1424 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1965,14 (Engl. Transl.)].
W. S. Johnson,Acc. Chem. Res., 1968, 1.
A. V. Semenovskii, D.Sci. (Chemistry) Thes., Institute of Organic Chemistry, USSR Acad. Sci., Moscow, 1972 (in Russian).
A. V. Semenovskii, V. A. Smit, and A. I. Dyadchenko,Izv. Akad. Nauk SSSR, Ser. Khim., 1974, 743 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1974,23 (Engl. Transl.)].
T. Mandai, M. Kawada, and J. Otera,J. Org. Chem., 1983,48, 5183.
PCMODEL, K.Gilbert, J.Gajewski, Serena Software, Bloomington, IN. (N. L. Allinger,J. Am. Chem. Soc., 1977,99, 8127).
S. Escher, W. Giersch, Y. Niclass, G. Bernardinelli, and G. Ohlaff,Helv. Chim. Acta., 1990, 1935.
D. K. Dalling and D. M. Grant,J.Am.Chem.Soc., 1972,94, 5318.
R. P. W. Kesselmans, J. B. P. A. Wijnberg, A. de Groot, and N. K. de Vries,J. Org. Chem., 1991,56, 7232.
M. Rance, O. W. SØrensen, B. Bodenhausen, G. Wagner, R. R. Ernst, and K. Wutrich,Biochem. Biophys. Res. Commun., 1983,17, 458.
J. Jeener, B. H. Meier, P. Bachmann, and R. R. Ernst,J. Chem. Phys., 1979,71, 4546.
A. Bax and S. Sabramanian,J. Magn. Reson., 1986,67, 565.
A. V. Buevich and Yu. A. Strelenko,Preceedigs of the 11-th International Meeting on NMR Spectroscopy, July 4–9, 1993, Swansea, UK.
M. Julia, C. Descoins, M. Baillarge, B. Jacquet, D. Uguen, and F. A. Groeger,Tetrahedron, 1975, 1737.
A. G. Andrewes, G. Borch, and S. Liaaen-Jensen,Acta Chem. Scand., 1984,38, 871.
R. Ratcliffe and R. Rodehorst,J. Org. Chem., 1970,35, 4000.
Author information
Authors and Affiliations
Additional information
Deceased.
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 156–163, January, 1994.
The authors are grateful to the representative of the Bruker company in Moscow, Uve Eichhof, who allowed us to use an AMX-400 spectrometer.
Rights and permissions
About this article
Cite this article
Moiseenkov, A.M., Lozanova, A.V., Surkova, A.A. et al. Synthesis and structure of tricyclic furanosesquiterpenoids related to pallescensin A. Russ Chem Bull 43, 153–160 (1994). https://doi.org/10.1007/BF00699157
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00699157