Abstract
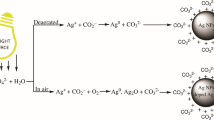
Colloidal copper has been obtained by γ-irradiation of aqueous solutions of copper (II) perchlorate in the presence of alcohol and polyethyleneimine (PEI). The sols are spherical particles about 4 nm in diameter, which are quickly oxidized by oxygen or other oxidants. When CuII is not entirely incorporated into the complex with PEI, disproportionation of CuI aqua complexes formed affords the metal, along with Cu2O. Reduction of the PEI complex of CuI by hydrated electrons gives only colloidal copper. The copper ions can be reduced on the surface of silver sols. Optical parameters of the resulting bimetallic particles have been studied. The presence of copper ions leads to broadening of the absorption band associated with the silver sols and shifts it to the UV region, which is due to the transfer of electrons from copper to silver. Three copper monolayers are enough to cause plasmon absorption of colloidal copper.
Similar content being viewed by others
References
B. G. Ershov,Usp. Khim., 1981,50, 2137 [Russ. Chem. Rews., 1981,50 (Engl. Transl.)].
B. G. Ershov, N. L. Sukhov, and M. A. Akinshin,Khim. vysok. energii, 1986,20, 392 [High Energy Chem., 1986,20 (Engl. Transl.)].
B. G. Ershov, E. Janata, M. Michaelis, and A. Henglein,J. Phys. Chem., 1991,95, 8996.
B. G. Ershov, E. Janata, and A. Henglein,Radiat. Phys. Chem., 1992,39, 123.
V. M. Latimer,Okislitel'nye sostoyaniya elementov i ikh potentsialy v vodnykh rastvorakh [Oxidation States of Elements and their Potentials in Aqueous Solutions], In. Lit., Moscow, 1954, 400 (Rus. Transl.).
G. Khyulst,Rasseyanie sveta malymi chastitsami [Light Scattering by Small Particles], In. Lit., Moscow, 1961, 536 (Rus. Transl.).
J. H. Weaver, C. Krafka, D. W. Lynch, and E. E. Koch,Optical Properties of Metals, Karlsruhe, 1981,1, 2.
J. A. Creighton and D. G. Eadon,J. Chem. Soc., Faraday Trans., 1991,87, 3881.
W. T. Doyle,Phys. Rev., 1958,111, 1067.
R. H. Doremus,J. Chem. Phys., 1965,42, 414.
A. Henglein, P. Mulvaney, T. Linnert, and A. Holzwarth,J. Phys. Chem., 1992,96, 2411.
A. Henglein, P. Mulvaney, A. Holzwarth, T. E. Socebee, and A. Fojtic,J. Phys. Chem., 1992,97, 21.
A. Henglein, M. Gutierrez, E. Janata. and B. G. Ershov,J. Phys. Chem., 1992,96, 4598.
B. G. Ershov and A. Henglein,J. Phys. Chem., 1993,97, 3434.
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 25–30, January, 1994.
Rights and permissions
About this article
Cite this article
Ershov, B.G. Colloidal copper in aqueous solutions: radiation-chemical reduction, mechanism of formation, and properties. Russ Chem Bull 43, 16–21 (1994). https://doi.org/10.1007/BF00699128
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00699128